Essential Corrosion Protection for Zinc Die Castings
TL;DR
Zinc die castings possess exceptional inherent corrosion resistance due to the formation of a stable, protective zinc oxide layer, often called a patina, which prevents the formation of red rust typical of iron-based metals. While this natural barrier is sufficient for many applications, its durability can be significantly enhanced for harsh or specific service environments. Advanced corrosion protection for zinc die castings is achieved through various surface treatments, including plating, chromate conversion coatings, and passivation, which provide added layers of defense against environmental threats.
Understanding Zinc's Natural Corrosion Resistance
The fundamental corrosion resistance of zinc die castings is not due to inertness but to a dynamic and protective reaction with the environment. Unlike ferrous metals that degrade by forming porous iron oxide (red rust), zinc alloys protect themselves through a process of oxidation. When a zinc die-cast part is exposed to air, its surface reacts with oxygen to form a thin, dense, and tightly adherent layer of zinc oxide. This initial layer is quite stable and slows down further oxidation significantly.
Over time, this zinc oxide layer continues to react with moisture and carbon dioxide in the atmosphere, forming a more complex and even more resilient layer of zinc carbonate. This combined layer, often referred to as a patina, is non-porous and self-healing to a degree. If the surface is scratched, the exposed zinc will simply re-oxidize and reform the protective barrier. This electrochemical process is the core reason why zinc is a superior choice for corrosion resistance in many applications. As explained by experts at Deco Products, this mechanism means zinc parts don't rust in the traditional sense; they form a protective shield.
However, this natural protection has its limits. While zinc alloys exhibit excellent performance in typical indoor and many outdoor environments, they can degrade over time, especially under prolonged exposure to aggressive conditions. According to insights from Dynacast, while aluminum alloys have a remarkable ability to self-heal, zinc will eventually break down. This makes it crucial to understand the specific environmental challenges a component will face and determine if its inherent resistance is sufficient or if it requires enhancement through secondary finishing processes.

Common Corrosion Threats: Understanding 'White Rust'
While zinc die castings do not form red rust, they are susceptible to a different form of corrosion known as "white rust." This phenomenon is a common concern for engineers and designers working with zinc alloys. White rust is a voluminous, white, powdery deposit that is primarily composed of zinc hydroxide. It forms when zinc surfaces are exposed to moisture, particularly in conditions with limited or no air circulation, which prevents the stable zinc carbonate patina from forming properly.
The chemical reaction for white rust is initiated when water (such as condensation, rain, or humidity) sits on the zinc surface. Without adequate airflow to dry the surface and provide carbon dioxide, the water reacts with the zinc to form zinc hydroxide instead of the more protective zinc oxide and carbonate layers. This often occurs when parts are tightly stacked, packed, or stored in damp, unventilated environments during shipping or warehousing. The trapped moisture creates an ideal microenvironment for the formation of these white, powdery deposits.
Although visually unappealing, white rust is often a superficial issue and does not typically indicate a rapid loss of structural integrity in the same way red rust does on steel. However, it can compromise the aesthetic quality of the part and, if left untreated, may interfere with the application of subsequent coatings or finishes. Preventing white rust is primarily a matter of proper handling and storage. Key preventative measures include:
- Ensuring parts are stored in a dry, well-ventilated area.
- Avoiding direct contact between parts during shipping by using spacers or appropriate packaging.
- Applying a temporary protective measure, such as a passivation treatment or conversion coating, if parts are expected to face high-humidity conditions.
Understanding the causes of white rust allows for the implementation of simple yet effective strategies to maintain the integrity and appearance of zinc die castings throughout their lifecycle.
A Guide to Protective Finishes for Zinc Die Castings
To augment zinc's natural corrosion resistance for more demanding applications, a wide array of surface finishes can be applied. These treatments not only provide an additional barrier against corrosive elements but can also enhance the part's appearance, wear resistance, and other functional properties. The selection of an appropriate finish depends on the service environment, aesthetic requirements, and cost considerations. Key methods include plating, conversion coatings, and passivation.
Plating involves depositing a thin layer of another metal onto the zinc die casting. Decorative chromium plating is a popular choice, providing a bright, reflective finish and excellent durability. As detailed by the International Zinc Association, for effective corrosion protection, it's critical that sufficient thicknesses of copper and nickel undercoats are applied before the final chromium layer. This multi-layer system creates a robust barrier against moisture and corrosive agents. Other metals like nickel and gold can also be used for plating, depending on the desired outcome.
Chromate Conversion Coatings are a chemical treatment that creates a thin, gel-like film on the surface of the zinc part. This film becomes an integral part of the surface and offers excellent corrosion resistance, particularly against the formation of white rust. Chromate coatings are available in various colors, including clear, blue, yellow, olive drab, and black, which can also serve as a final finish. They are also an excellent primer for paint and powder coatings, significantly improving adhesion.
Passivation is another chemical process that enhances corrosion resistance by removing free iron and other contaminants from the surface, creating a passive oxide layer. As described by Diecastor, this process is highly effective at preventing surface corrosion and maintaining a clean appearance. It is often used as a final step to protect parts during storage and transit or as a standalone protective finish for less severe environments.
To aid in the selection process, the following table compares these common protective finishes:
| Finish Type | Corrosion Resistance | Appearance | Relative Cost | Common Applications |
|---|---|---|---|---|
| Plating (e.g., Chrome) | Very High | Bright, reflective, decorative | High | Automotive trim, plumbing fixtures, decorative hardware |
| Chromate Conversion Coating | High | Varies (clear, yellow, black) | Low to Medium | Electronic components, fasteners, under-paint primer |
| Passivation | Medium | Clear, maintains original look | Low | General protection for shipping, moderate environments |
| Powder Coating / Painting | High | Wide range of colors and textures | Medium | Housings, consumer products, architectural parts |
Comparative Analysis: Zinc vs. Other Die Casting Alloys
When designing a component, selecting the right material is the first and most critical step in ensuring long-term performance and corrosion resistance. While zinc alloys offer an excellent balance of properties, it is valuable to compare them against other common die casting materials like aluminum and magnesium.
Zinc vs. Aluminum: Both zinc and aluminum alloys are known for their corrosion resistance, but they achieve it through different mechanisms. As previously discussed, zinc forms a protective patina. Aluminum also forms a protective oxide layer that is highly effective and self-healing. According to Compass & Anvil, aluminum's lightweight nature and ability to withstand high temperatures make it a versatile choice. Zinc, however, offers superior castability, allowing for thinner walls, tighter tolerances, and smoother surface finishes straight from the die, which can reduce or eliminate the need for secondary machining operations. The choice often comes down to the specific application's need for strength, weight, thermal properties, and precision.
Zinc vs. Magnesium: Magnesium is the lightest of all structural metals, offering an exceptional strength-to-weight ratio. However, it is not inherently corrosion-resistant and typically requires a protective coating or spray to prevent galvanic corrosion, especially in damp or marine environments. Zinc provides far superior natural corrosion resistance, making it a more straightforward choice for parts exposed to the elements without additional surface treatments.
For demanding sectors like the automotive industry, where components must be both robust and precisely manufactured, material selection is paramount. Companies specializing in high-performance components, such as Shaoyi (Ningbo) Metal Technology, leverage advanced processes like hot forging to create precision-engineered automotive parts that meet stringent quality and durability standards. Their expertise in producing components from prototyping to mass production highlights the importance of matching advanced materials with sophisticated manufacturing techniques to achieve optimal performance.
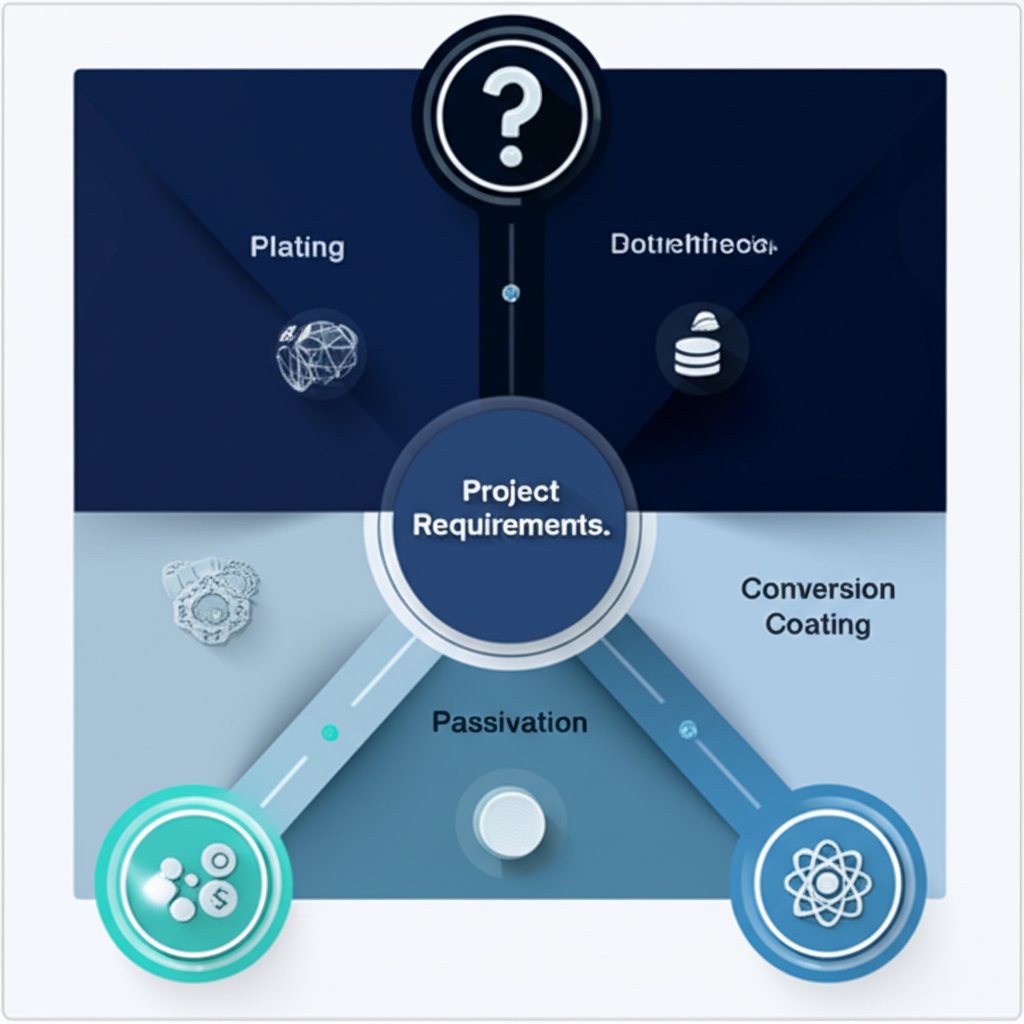
Selecting the Optimal Protection Strategy
Ultimately, achieving the desired longevity for a zinc die-cast component requires a holistic approach. The decision-making process should begin with a thorough analysis of the part's intended service environment. For components used in controlled, indoor settings, the natural corrosion resistance of the zinc alloy may be entirely sufficient. In these cases, focusing on a clean, as-cast finish can be the most cost-effective solution.
For parts exposed to humidity, intermittent moisture, or outdoor atmospheric conditions, an additional layer of protection is warranted. A chromate conversion coating or passivation treatment offers a significant upgrade in durability with minimal cost, effectively preventing the onset of white rust and preserving the part's appearance. For the most aggressive environments—such as marine applications, industrial settings with chemical exposure, or components requiring high wear resistance—a multi-layer plating system or a robust powder coat is the most reliable strategy. By carefully matching the material's inherent properties with a tailored surface finish, engineers can ensure that zinc die castings deliver exceptional performance and durability across a vast range of applications.
Frequently Asked Questions
1. Is die cast zinc corrosion resistant?
Yes, zinc die casting alloys are inherently corrosion resistant. They react with oxygen and carbon dioxide in the air to form a stable, non-porous protective layer known as a patina. This layer prevents the formation of red rust and shields the underlying metal from further corrosion. While this natural protection is excellent, it can be enhanced with coatings for very harsh environments.
2. What is the anti-corrosion method that uses zinc?
The most common anti-corrosion method that uses zinc to protect other metals (primarily steel) is called galvanizing. In this process, a steel part is coated with a layer of zinc. The zinc acts as a sacrificial barrier, corroding preferentially to protect the steel underneath. This is distinct from protecting a zinc die casting itself, which relies on its own patina or applied surface finishes.
3. How can you stop zinc from tarnishing?
Tarnishing on zinc is the formation of its natural oxide/carbonate patina, which dulls the initial bright finish. To prevent this for aesthetic reasons, or to stop the formation of white rust, a protective coating is necessary. Clear lacquers, waxes, passivation treatments, or chromate conversion coatings can seal the surface from the atmosphere, preserving its appearance and adding a layer of protection.
4. How is zinc inherently corrosion resistant?
Zinc's inherent corrosion resistance comes from its electrochemical properties. It has a natural ability to form corrosion byproducts—specifically zinc oxide and later zinc carbonate—that create a passive, tightly-adhering protective barrier on its surface. This patina is stable and significantly reduces the rate of further corrosion, effectively shielding the metal from environmental factors.
 Small batches, high standards. Our rapid prototyping service makes validation faster and easier —
Small batches, high standards. Our rapid prototyping service makes validation faster and easier — 
