Charge of Aluminum Explained: From Electron Shells to Al3+

Quick answer and the concepts you should not mix up
Quick answer: Aluminum’s most common ionic charge
Aluminum typically forms a +3 ion (Al3+). For most chemistry questions, the charge of aluminum is +3. In covalent contexts, we discuss oxidation states; surface or electrostatic charge is a different concept. Don’t confuse these terms—Al3+ is your answer for nearly all general chemistry problems.
Why this is the accepted charge in general chemistry
When you see a question like “what is the charge of aluminum,” the answer is almost always +3. This is because aluminum atoms lose three electrons to reach a stable, noble-gas electron configuration. The resulting ion, Al3+, is called the aluminum ion and is the form found in compounds like aluminum oxide and aluminum chloride. This convention is recognized by IUPAC and is reflected in standard chemical references.
Don’t mix up these three concepts
- Ionic charge: The actual charge on an aluminum ion (Al3+) found in salts and ionic compounds. This is what most chemistry questions mean by “charge of an aluminum ion.”
- Oxidation state: A formal bookkeeping number used to track electron transfers in reactions. For aluminum, the oxidation state is usually +3 in compounds, but in rare organometallics, it can be lower (see advanced chemistry sections).
- Surface/electrostatic charge: The net electrical charge on a piece of metallic aluminum, which can vary depending on its environment (e.g., in electrochemistry or at interfaces). This is a physical property, not the same as ionic or oxidation charge.
When exceptions appear and why they’re rare
Are there exceptions to the +3 rule? Yes—but only in highly specialized, advanced chemistry. Lower oxidation states of aluminum can be found in some organometallic compounds, but these are not encountered in general chemistry or everyday applications. For nearly all practical and educational purposes, +3 is the accepted charge (IUPAC guidelines).
What’s next? If you want to understand why +3 is so stable, keep reading to learn how aluminum’s electron configuration and ionization energies make Al3+ the dominant species. Later, we’ll see how this charge shows up in real compounds, and why surface charge is a different story entirely.
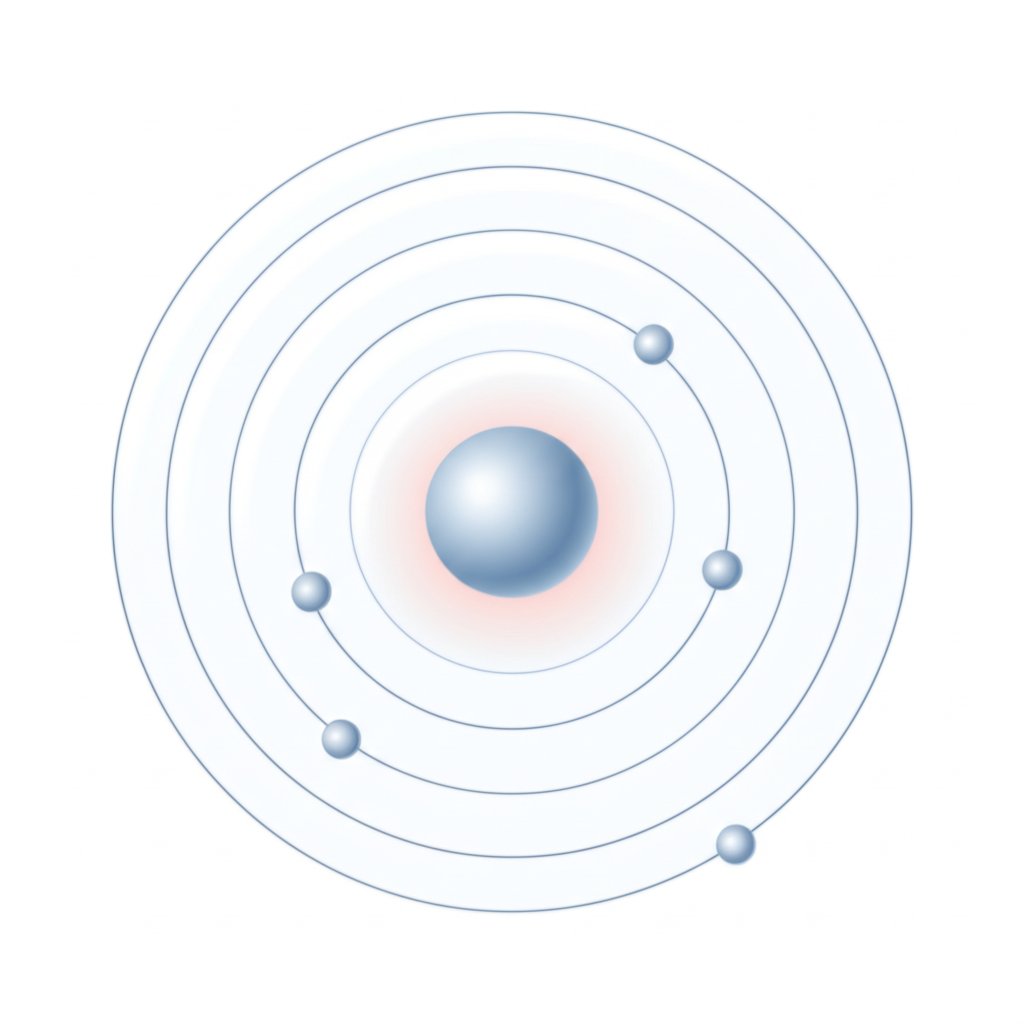
How electron configuration leads to Al3+ step by step
Electron configuration that drives Al3+
Ever wondered why aluminum almost always shows up as Al3+ in chemistry problems? The answer lies in its electron configuration. When you ask, “how many electrons does aluminum have?” in its neutral state, the answer is 13. These electrons are arranged into specific shells and subshells, following a predictable order based on energy levels.
Here’s the full breakdown for a neutral aluminum atom (LibreTexts):
1s2 2s2 2p6 3s2 3p1
This configuration tells you that aluminum’s valence electrons—the electrons available for bonding or removal—are in the third shell (n=3): two in 3s and one in 3p. That’s three valence electrons in total. So, if you’re asked, “how many valence electrons does aluminum have?” or “what are the al valence electrons?” the answer is three: 3s23p1.
From neutral atom to cation in three clean steps
Let’s walk through how aluminum becomes Al3+—an aluminum ion with 10 electrons—step by step:
- Start with the neutral atom: 13 electrons arranged as shown above.
- Remove the highest-energy electron first: The single 3p electron is lost, leaving 3s2.
- Remove the next two highest-energy electrons: Both 3s electrons are removed, leaving only the 1s2 2s2 2p6 configuration.
After these three electrons are removed, you’re left with 10 electrons—the same as neon, a noble gas. That’s why the aluminum ion with 10 electrons is so stable: it has a filled shell, just like a noble gas.
| Species | Electron Configuration | Number of Electrons |
|---|---|---|
| Neutral Al atom | 1s2 2s2 2p6 3s2 3p1 | 13 |
| Al3+ ion | 1s2 2s2 2p6 | 10 |
Why losing three electrons is favored over other options
Why doesn’t aluminum stop at losing just one or two electrons? The answer comes from stability. After losing three, aluminum achieves a noble-gas core (like Ne), which is especially stable. If it only lost one or two electrons, the resulting ions would have partially filled shells, which are much less stable and rarely observed in basic chemistry.
Removing three valence electrons yields Al3+ with a stable core; that’s why +3 dominates basic inorganic chemistry.
Common pitfalls when working with aluminum electron configurations
- Don’t remove electrons from the 2p subshell—only the outermost (3p and 3s) electrons are lost first.
- Avoid mixing up the order: 3p electrons are removed before 3s electrons.
- Remember: the number of valence electrons in aluminum is three—not one, not two.
- Double-check your total: after forming Al3+, you should have an aluminum ion with 10 electrons.
Understanding this stepwise process helps explain why Al3+ is energetically favored—a topic we’ll connect to ionization energies in the next section.
Why Al3+ Dominates: The Ionization Energy Perspective
First, Second, and Third Ionizations Versus the Fourth
When you wonder why the ion charge of aluminum is almost always +3, the answer lies in the energy required to remove electrons—known as ionization energy. Imagine you’re peeling away layers from an onion: the outer layers come off easily, but once you reach the core, it gets much tougher. The same principle applies to aluminum atoms.
Let’s break it down. Aluminum starts with three valence electrons in its outer shell. Removing the first electron (IE1), then the second (IE2), and the third (IE3) are all relatively feasible because these electrons are farther from the nucleus and shielded by inner electrons. But removing a fourth electron (IE4) means breaking into a stable, closed-shell core—this requires a massive jump in energy.
| Ionization Step | Which Electron Is Removed? | Relative Energy Cost |
|---|---|---|
| IE1 | First valence (3p1) | Moderate |
| IE2 | Second valence (3s1) | Moderate |
| IE3 | Third valence (3s1) | Still manageable |
| IE4 | Core electron (2p6) | Huge jump |
According to published data (Lenntech), aluminum’s first ionization energy is about 5.99 eV, but the energy required for the fourth electron skyrockets. This steep increase is why aluminum virtually never forms +4 ions in nature. So, does Al gain or lose electrons to become stable? It loses electrons—specifically, three valence electrons—before the cost becomes prohibitive.
Stability After Three Electrons Are Removed
What happens when aluminum has lost those three electrons? You’re left with an aluminum ion (Al3+) that has a noble gas electron configuration, matching neon. This configuration is exceptionally stable, so aluminum “stops” at a +3 charge. This is why, if you’re asked, “does aluminum have a fixed charge?” in most chemistry contexts, the answer is yes—+3 is the only common al ionic charge you’ll encounter.
But what about the electron affinity of aluminum? This value is relatively low, meaning aluminum doesn’t easily gain electrons back after forming Al3+. The process is energetically one-way: lose three electrons, reach a stable state, and stay there.
A sharp ionization energy jump after the third electron explains the dominance of Al3+.
Practical Implications: Why Al3+ Matters in Chemistry and Industry
- Common +3 salts: Compounds like aluminum oxide (Al2O3) and aluminum chloride (AlCl3) always feature aluminum in the +3 state.
- Hydrolysis and water chemistry: The ionic charge for aluminum governs how Al3+ ions interact with water, leading to hydrolysis and precipitation of aluminum hydroxide. (See the next section for real-world water chemistry.)
- Minerals and materials: Aluminum’s +3 charge is the foundation for mineral structures like alumina and for the formation of protective oxide layers that prevent corrosion.
So, the next time you wonder, “does aluminum have a fixed charge?” or “why doesn’t aluminum form +1 or +2 ions?” you’ll know the answer is all about the steep rise in ionization energy after three electrons are gone. The +3 state is energetically favored and chemically reliable.
The energetic cliff beyond the third electron removal underpins aluminum’s strong tendency to form Al3+.
Ready to see how this charge plays out in real-world water chemistry and industrial applications? The next section explores aluminum’s behavior in aqueous solutions and why its +3 charge is so important for both science and technology.
Ionic charge and oxidation state versus surface charge
Ionic or oxidation charge in compounds
When you see a question like “what is the aluminium ionic charge in Al2O3 or AlCl3?”, you’re dealing with oxidation states and ionic charges—not the physical charge of a metal surface. In simple ionic compounds, the charge on aluminum is +3, matching its oxidation state. For example, in aluminum oxide, each Al atom is considered to have lost three electrons, becoming Al3+, while each oxygen is O2−. This “+3” is a formal bookkeeping tool that helps chemists track electron transfers and balance reactions (LibreTexts Redox).
In summary, the ionic aluminum charge is always +3 in general chemistry contexts. This is distinct from any transient or physical charge found on a piece of bulk aluminum metal.
Surface and electrostatic charge on bulk aluminum
Now imagine you’re holding a piece of aluminum foil. The net charge on its surface—called surface or electrostatic charge—can fluctuate based on its environment. For example, if you rub aluminum against another material, or expose it to a high-voltage field, you can build up a temporary static charge. In electrochemical setups, the surface charge density can be measured with specialized tools, and is influenced by adsorbed water, oxide films, and even air humidity.
But here’s the catch: the surface charge is not the same as the ionic charge in a compound. The two concepts are measured differently, have different units, and answer different types of questions.
| Aspect | Ionic/Oxidation Charge | Surface/Electrostatic Charge |
|---|---|---|
| Definition | Formal charge assigned to Al in compounds (e.g., +3 in Al3+ or Al2O3) | Physical net charge on the surface of bulk aluminum metal |
| Units | Elementary charge (e), or simply “+3” | Coulombs (C), or C/m2 for charge density |
| Where it’s measured | In chemical formulas, reactions, and stoichiometry | On actual aluminum surfaces; varies with environment |
| Tools used | Stoichiometry, titration, oxidation-state rules | Kelvin probe, zeta potential, surface voltmeters |
| Typical classroom question | “What is the charge of an aluminum ion?” "What is the oxidation state of Al in Al2O3?" | “How does a charged Al surface behave in electrolyte?” "How much static charge is on this foil?" |
Why confusion leads to wrong answers
Sounds complex? Not really, once you keep the distinction clear. Many students mix up the aluminum ions found in compounds with the temporary charge that can build up on a metal’s surface. For example, a chemistry test might ask about the “charge on aluminum” in AlCl3—here, you’re expected to answer +3, not a value in coulombs.
In practical terms, the surface charge on aluminum is usually neutralized quickly by air or water. But in certain conditions—like high-voltage experiments, or friction between materials—surface charge can build up and be measured. This is especially important in triboelectric and electrostatic applications (Nature Communications).
One more thing: you might wonder, “will aluminum rust if it’s carrying a surface charge?” The answer is that aluminum does not rust like iron does, because rusting refers specifically to iron oxide. Instead, aluminum forms a thin, protective oxide layer that shields it—even if there’s a temporary surface charge present. So, if you’re worried about whether aluminum will rust, rest assured: it won’t, but it can corrode under harsh conditions, and surface charge plays little role in that process.
Oxidation state is chemistry bookkeeping; surface charge is a physical surface property.
- “What is the charge of an aluminum ion?” → Answer: +3 (oxidation/ionic charge)
- “How does a charged Al surface behave in electrolyte?” → Answer: Depends on surface charge, environment, and measurement method
- “Will aluminum rust if exposed to water?” → No, but it may corrode; the oxide layer prevents rusting
Keeping these concepts clear will help you ace chemistry questions and avoid common mistakes. Next, we’ll see how to apply oxidation-state rules to real compounds—so you can determine aluminum’s charge with confidence every time.
Worked examples determining aluminum oxidation states
Classic salts: Step-by-step oxidation state calculations for Al2O3 and AlCl3
Ever wondered how chemists figure out the ionic charge aluminum takes in common compounds? Let’s walk through the process with classic examples, using simple rules and a stepwise approach you can use on any test or in the lab.
Example 1: Aluminum oxide (Al2O3)
- Assign known oxidation states: Oxygen is almost always −2 in simple compounds.
-
Set up the sum-to-zero equation:
- Let x = oxidation state of Al
- 2(x) + 3(−2) = 0
-
Solve for Al:
- 2x − 6 = 0
- 2x = 6
- x = +3
Conclusion: The charge for aluminum in Al2O3 is +3, matching the formula for aluminium ion in most general chemistry scenarios. The ion name for aluminum here is "aluminum(III) ion" or simply "aluminum ion."
Example 2: Aluminum chloride (AlCl3)
- Assign known oxidation states: Chlorine is almost always −1.
-
Set up the sum-to-zero equation:
- Let x = oxidation state of Al
- x + 3(−1) = 0
-
Solve for Al:
- x − 3 = 0
- x = +3
So, the alcl3 charge for each aluminum is +3 as well. You’ll notice this pattern in almost every simple salt containing aluminum.
Beyond basics: Aluminum sulfide and hydroxo complexes
Example 3: Aluminum sulfide (Al2S3)
- Assign known oxidation states: Sulfur is −2 in sulfides.
-
Set up the sum-to-zero equation:
- Let x = oxidation state of Al
- 2x + 3(−2) = 0
-
Solve for Al:
- 2x − 6 = 0
- 2x = 6
- x = +3
The aluminum sulfide formula (Al2S3) always features Al in the +3 state. This confirms the aluminum charge ion is +3, just as in oxides and chlorides.
Example 4: Coordination complex K[Al(OH)4]
- Determine the charge of the complex ion: Potassium (K) is +1, so the complex ion must be −1.
- Assign known oxidation states: Hydroxide (OH⁻) is −1 for each group.
-
Set up the sum-to-ion-charge equation for [Al(OH)₄]⁻:
- Let x = oxidation state of Al
- x + 4(−1) = −1
- x − 4 = −1
- x = +3
Even in this hydroxocomplex, aluminum keeps its usual +3 oxidation state. The negative charge is carried by the extra hydroxide ligand, not by lowering Al’s oxidation state.
Check your work: Sum rules and common mistakes
- Always double-check that the sum of all oxidation numbers equals the net charge of the molecule or ion.
- Remember: in neutral compounds, the sum is zero; in ions, it equals the ion’s charge.
- Use the periodic table to recall common anion charges (O is −2, Cl is −1, S is −2, OH is −1).
- For polyatomic ions, calculate the sum inside the brackets first, then assign the charge outside.
- Consult IUPAC oxidation state guidelines for edge cases.
If you know the common anion charges, Al almost always balances to +3 in inorganic salts.
Practice: Can you solve these?
- What is the oxidation state of Al in Al(NO3)3?
- Determine the charge for aluminum in Al2(SO4)3.
- Find the oxidation state of Al in [Al(H2O)6]3+.
Answers:
- Al(NO3)3: Nitrate is −1, three nitrates is −3; Al is +3.
- Al2(SO4)3: Sulfate is −2, three sulfates is −6; two Al must total +6, so each Al is +3.
- [Al(H2O)6]3+: Water is neutral, so Al is +3.
Mastering these steps will help you confidently determine the ionic charge aluminum takes in any compound, and avoid common pitfalls with the formula for aluminium ion or the ion name for aluminum. Up next, we’ll see how these oxidation states play out in water and real-world reactions.

Aqueous chemistry and amphoterism of Al3+ in practice
Hydrolysis to Al(OH)3 and formation of aquo complexes
When aluminum enters water as Al3+—the classic aluminum ion charge—its journey is anything but static. Imagine pouring an aluminum salt into water: the Al3+ ions don’t just float around as bare ions. Instead, they quickly attract water molecules, forming hydrated complexes like [Al(H2O)6]3+. This hydrated symbol for aluminum ion is the starting point for a series of fascinating reactions that depend on pH.
As you raise the pH (make the solution less acidic), the Al3+ ion begins to hydrolyze—meaning it reacts with water to form aluminum hydroxide, Al(OH)3. This process is visible in lab tests as the formation of a white, gelatinous precipitate. According to USGS research, at neutral to slightly basic pH (around 7.5–9.5), this precipitate is often amorphous at first but can age into more crystalline forms like gibbsite or bayerite (USGS Water Supply Paper 1827A).
Amphoterism: Dissolving in acids and bases
Now, here’s where things get interesting. Aluminum hydroxide, Al(OH)3, is amphoteric. That means it can react with both acids and bases. In acidic solutions, Al(OH)3 dissolves back into Al3+ ions. In strongly basic solutions, it reacts with excess hydroxide to form soluble aluminate ions, [Al(OH)4]−. This dual behavior is what makes aluminum so versatile in water treatment and environmental chemistry (Anal Bioanal Chem, 2006).
So, how does an atom of aluminum become an ion in water? It loses three electrons, forming Al3+, which then interacts with water molecules and undergoes hydrolysis or complexation based on the surrounding pH. This process is a textbook example of how aluminum lose or gain electrons to adapt to its environment, but in practice, it always loses electrons to become an ion.
pH-dependent speciation: What dominates where?
Wondering which species you’ll find at different pH levels? Here’s a simple guide:
- Acidic region (pH < 5): Dominated by hydrated aluminum ions, [Al(H2O)6]3+. The solution is clear, and aluminum cation or anion speciation is simple—just Al3+.
- Neutral region (pH ~6–8): Hydrolysis leads to precipitation of Al(OH)3 (s), a white solid. This is the classic aluminum hydroxide floc used in water purification.
- Basic region (pH > 9): Al(OH)3 dissolves to form aluminate ions, [Al(OH)4]−, which are transparent and highly soluble.
This pH-dependent behavior is crucial for understanding how aluminum gain or lose electrons in different chemical environments. For example, in acidic lakes or soils, aluminum stays dissolved—posing environmental risks. In neutral water, it precipitates out, and in alkaline conditions, it stays dissolved again but as a different species.
Why amphoterism matters in real life
Why should you care about all this chemistry? Amphoterism underpins aluminum’s role in water treatment, where Al3+ salts are used to remove impurities by forming sticky flocs of Al(OH)3. It also explains why aluminum resists corrosion in many environments but can dissolve in both strong acids and bases. In cleaning chemistry, the ability of aluminum to react with both acids and bases allows for tailored solutions to remove deposits or passivate surfaces.
Aluminum’s +3 center hydrolyzes, precipitates, and forms aluminate in base—classic amphoterism in action.
- Acidic: [Al(H2O)6]3+ (soluble, clear)
- Neutral: Al(OH)3 (s) (precipitate, floc)
- Basic: [Al(OH)4]− (soluble, clear)
So, the next time you’re asked, “what is the charge of an aluminum ion in water?” or “is aluminum cation or anion?”—you’ll know that the answer depends on pH, but the underlying theme is always the loss of electrons to form Al3+, followed by hydrolysis and amphoteric transformations (USGS).
Understanding these aqueous behaviors not only helps in chemistry class but also connects to environmental science, engineering, and even public health. Up next, we’ll see how these charge concepts translate into real-world materials and manufacturing, from corrosion resistance to the creation of high-performance aluminum components.

From chemistry to manufacturing and trusted extrusion sources
From Al3+ in compounds to oxide-protected metal surfaces
Ever wondered how the charge of aluminum translates from chemistry class to real-world products? The answer starts with the surface. The moment a piece of aluminum is exposed to air, it reacts rapidly with oxygen to form a thin, invisible layer of aluminum oxide (Al2O3). This layer is only a few nanometers thick but is incredibly effective at protecting the underlying metal from further corrosion. Unlike iron, which forms flaky rust, aluminum’s oxide is self-sealing and tenacious—so, if you’ve ever asked, “will aluminum rust?” the answer is no. Aluminum does not rust like iron; instead, it passivates, creating a stable barrier that prevents ongoing degradation.
This protective oxide is more than just a shield—it’s a direct result of aluminum’s +3 charge in compounds. In Al2O3, each aluminum atom is bonded ionically to oxygen, contributing to the material’s high hardness and wear resistance. That’s why aluminum oxide is used in sandpaper and cutting tools, and why aluminum extrusions for automotive or aerospace use can last for decades without structural compromise.
Why extrusion, forming, and finishing depend on surface chemistry
Imagine you’re designing a car part or an outdoor structure. You’ll notice that aluminum comes in many forms: sheet, plate, channel, and especially aluminum extrusion parts. Each form relies on the stability of the oxide layer for performance—but that same layer can also affect manufacturing steps like welding, bonding, or finishing.
- Anodizing: This process thickens the natural oxide, improving corrosion resistance and allowing for vibrant colors or matte textures. The quality of anodizing depends on alloy composition and surface prep.
- Bonding & Sealing: Adhesive bonding works best on freshly cleaned aluminum, as the oxide layer can hinder some adhesives if not properly prepared. For sealing, the oxide enhances paint and powder-coat adhesion, helping parts withstand weathering.
- Welding: The oxide must be removed before welding, because it melts at a much higher temperature than the metal itself. Failing to do so leads to weak joints and defects.
Understanding amphoterism—the ability of aluminum hydroxide to react with both acids and bases—guides pretreatments. For example, alkaline or acidic cleaning steps are used to remove contaminants and condition the oxide before finishing. This ensures that the final product has consistent appearance and maximum durability.
The invisible oxide layer formed due to aluminum’s +3 charge is the secret to its durability and resistance to corrosion—making it the backbone of reliable manufacturing, not just a chemistry curiosity.
Where to source precision automotive extrusions
When it comes to advanced manufacturing—especially for automotive, aerospace, or architectural projects—choosing the right aluminum extrusion supplier is critical. Not all extrusions are created equal: the quality of the alloy, the consistency of the oxide layer, and the precision of forming and finishing operations all impact the final product’s performance and appearance.
- Sheet and plate: Used for body panels, chassis, and enclosures; surface finish is critical for painting and sealing.
- Channels and profiles: Found in structural frames and trim, where anodizing or powder coating can enhance durability.
- Custom extrusions: Automotive suspension, battery enclosures, or lightweight structural parts—where strict tolerances and traceable quality are non-negotiable.
For those seeking a partner who understands both the science and the engineering, Shaoyi Metal Parts Supplier stands out as a leading integrated provider of precision aluminum extrusion parts in China. Their expertise covers every step, from alloy selection and extrusion to surface treatment and quality control. By leveraging a deep understanding of aluminum’s charge-driven surface chemistry, they deliver components that excel in corrosion resistance, bonding, and long-term reliability.
So, the next time you hear someone ask, “what is the charge on aluminum?” or “will aluminum rust in real-world use?”—you’ll know the answer is rooted in both chemistry and engineering. The protective oxide layer, born from aluminum’s +3 charge, is your assurance of durability—whether you’re designing a car, building, or any high-performance product.
Key takeaways and a practical next step
Key takeaways you can recall in seconds
Let’s bring it all together. After exploring the charge of aluminum from electron shells to real-world manufacturing, you might wonder: what is aluminums charge, and why does it matter so much? Here’s a quick checklist to cement your understanding and help you ace any chemistry or engineering question about aluminum:
- Al3+ is the canonical ionic charge: In almost all general chemistry and industrial contexts, the answer to "what is the ion charge of aluminum" is +3. This is the form found in salts, minerals, and most compounds (Echemi: Charge of Aluminum).
- Electron configuration explains +3: Aluminum has 13 electrons; it loses three valence electrons to achieve a stable, noble-gas-like core. This makes Al3+ particularly stable and common.
- Ionization energy sets the limit: The energy needed to remove a fourth electron is prohibitively high, so aluminum stops at +3. This is why, if you’re asked "what charge does aluminum have" in a salt or solution, the answer is always +3.
- Oxidation state vs surface charge: Don’t confuse the formal oxidation state (+3 in most compounds) with the physical surface charge on metallic aluminum. The former is a chemistry bookkeeping tool; the latter is a property of bulk metal and its environment.
- Aqueous amphoterism is key: Aluminum’s +3 center can hydrolyze, precipitate, or form aluminate ions depending on pH—a classic example of amphoterism in action.
Think ‘valence to noble-core’—that logic gets you to Al3+ fast in most problems.
Where to read more and apply the knowledge
If you want to dig deeper into what is aluminum charge and its broader implications, here are some excellent resources:
- IUPAC Oxidation State Guidelines – For precise definitions and conventions on oxidation numbers.
- NIST Chemistry WebBook: Aluminum – For authoritative atomic and ionization data.
- Standard inorganic chemistry textbooks – For stepwise explanations, worked examples, and further applications in materials science.
Apply your new knowledge by analyzing the charge of Al in unfamiliar compounds, predicting reactivity in water, or understanding why certain alloys and surface treatments work so well in manufacturing.
Smart next step for engineered extrusions
Ready to see how this chemistry shapes real-world products? When sourcing or designing automotive, aerospace, or construction components, understanding what is al charge helps you select the right materials, surface treatments, and manufacturing processes. For precision-engineered aluminum extrusion parts, partnering with an expert like Shaoyi Metal Parts Supplier ensures that every aspect—from alloy selection to oxide layer management—is optimized for durability, joining, and corrosion protection. Their expertise in aluminum’s charge-driven surface chemistry means you get components that perform reliably in demanding environments.
Whether you’re a student, engineer, or manufacturer, mastering the charge of al is your key to making smarter choices in both chemistry and industry. Next time someone asks, "what is aluminums charge?" or "what is al charge?"—you’ll have the answer, and the reasoning, at your fingertips.
Frequently Asked Questions about the Charge of Aluminum
1. Why does aluminum have a +3 charge in most compounds?
Aluminum typically has a +3 charge because it loses its three valence electrons to achieve a stable, noble gas electron configuration. This makes Al3+ highly stable and the most common ionic form found in compounds like aluminum oxide and aluminum chloride.
2. Is the charge of aluminum always +3 or are there exceptions?
While +3 is the standard charge for aluminum in most chemical compounds, rare exceptions exist in advanced organometallic chemistry where aluminum can display lower oxidation states. However, these cases are not common in general chemistry or everyday applications.
3. How does the electron configuration of aluminum lead to its +3 charge?
Aluminum has 13 electrons, with three in its outermost shell (valence electrons). It loses these three electrons to form Al3+, resulting in a stable electron configuration matching that of neon, a noble gas. This stability drives the preference for a +3 charge.
4. Does aluminum rust like iron, and how does its charge affect corrosion?
Aluminum does not rust like iron because it forms a thin, protective oxide layer (Al2O3) that prevents further corrosion. This layer is a direct result of aluminum's +3 charge in compounds, providing long-term durability in real-world applications.
5. Why is understanding the charge of aluminum important in manufacturing?
Knowing that aluminum forms a +3 charge explains its surface chemistry, corrosion resistance, and suitability for processes like anodizing and bonding. This knowledge is crucial for selecting materials and treatments in automotive and industrial manufacturing, ensuring reliable, high-quality aluminum components.
 Small batches, high standards. Our rapid prototyping service makes validation faster and easier —
Small batches, high standards. Our rapid prototyping service makes validation faster and easier — 
