Aluminium Ionic Charge: Predict + Balance Formulas In Seconds
Aluminium Ionic Charge at a Glance
Quick answer: what charge does aluminium form?
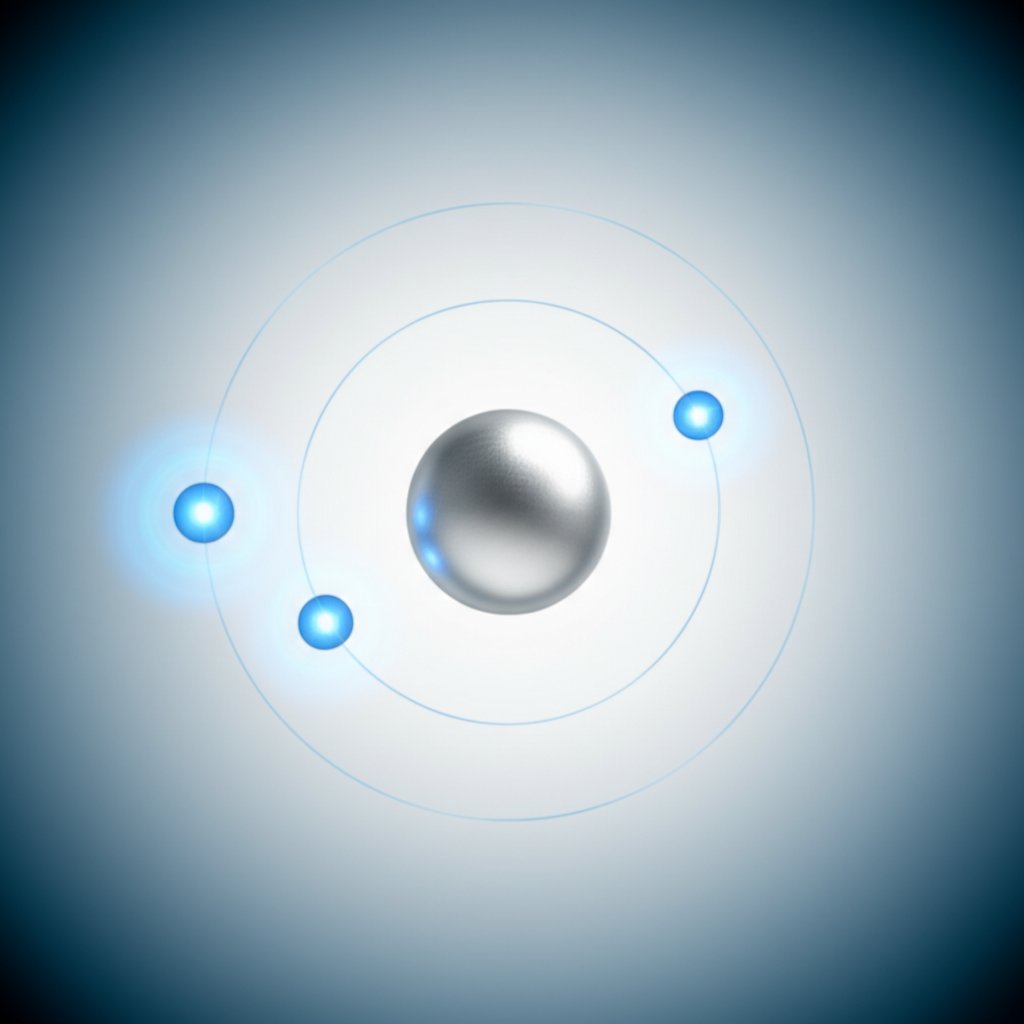
If you’re looking for the short version, here it is: aluminium nearly always forms an ion with a +3 charge. In chemical terms, this is written as Al3+. That’s the most common—and most stable—aluminum ion you’ll encounter in compounds, from everyday materials to industrial applications.
Typical aluminium ionic charge is +3 (Al3+).
Why is this the case? The secret lies in aluminium’s position on the periodic table and its atomic structure. Aluminium (Al) is found in group 13, where each neutral atom has three valence electrons. When aluminium reacts to form an ion, it loses those three outermost electrons, resulting in a net positive charge of +3. This process is summed up in a single half-reaction:
Al → Al3+ + 3e−
So, when you see the phrase aluminium ionic charge or wonder what is the charge of aluminum, you’re really asking how many electrons aluminium loses to become stable. The answer: three. That’s why the charge of an aluminum ion is almost always +3 in salts and solutions.
- Pairs with anions totaling −3: Al3+ combines with negative ions to balance its charge, like two Al3+ for three O2− in Al2O3.
- Predictable formulas: Compounds such as Al2O3 (aluminum oxide) and AlCl3 (aluminum chloride) reflect this +3 charge.
- Strong lattice formation: The +3 charge leads to robust ionic lattices, giving aluminum compounds their stability and usefulness in materials.
It’s important to note that “ionic charge” refers specifically to the net charge after aluminium has lost electrons—not to be confused with terms like oxidation number or valence (we’ll clarify those in a later section). For now, just remember: if you’re asked about the aluminum ion charge, the answer is +3.
Ready to see how you can predict this charge for any element, not just aluminium? In the next section, you’ll get a step-by-step guide to reading the periodic table, understanding why Al3+ is so reliable, and applying this knowledge to write balanced chemical formulas. We’ll also break down the energetic “why,” compare related concepts, and give you hands-on practice problems with solutions. Let’s get started!
Predicting Ionic Charge with Confidence
How to know the charge of an element using periodic trends
Ever wondered if there’s a shortcut to predict the ionic charge of an atom just by glancing at the periodic table? Good news: there is! The periodic table is more than a list of elements—it’s a powerful tool for learning how to know the charge of an element and for predicting the charges of elements in their most common ionic forms. Here’s how you can use it to your advantage, whether you’re working with aluminium, magnesium, oxygen, or others.
- Find the element’s group number. The group (vertical column) often tells you how many valence electrons the element has. For main-group elements, the group number is key.
- Decide if the element is a metal or nonmetal. Metals (left side of the periodic table) tend to lose electrons and form positive ions (cations). Nonmetals (right side) usually gain electrons to become negative ions (anions).
-
Apply the main rule:
- For metals: The ionic charge is typically equal to the group number (but positive).
- For nonmetals: The ionic charge is the group number minus eight (resulting in a negative charge).
- Double-check with common compounds and stability trends. The most common charge for an element lines up with the formulas of its stable compounds.
Periodic cue: Left side metals → cations; right side nonmetals → anions. Transition metals (center block) are more variable, but main-group elements follow these patterns closely.
Apply the rules: aluminium, magnesium, and oxygen
- Aluminium (Al): Group 13 metal. Loses three electrons to form Al3+. This is the classic aluminium ionic charge.
- Magnesium (Mg): Group 2 metal. Loses two electrons to form Mg2+—the standard magnesium ion charge.
- Oxygen (O): Group 16 nonmetal. Gains two electrons to form O2−, a common anion.
Let’s see these predictions in action with quick examples:
- Aluminium (Al): Group 13 → loses 3 electrons → Al3+ (aluminum ion)
- Magnesium (Mg): Group 2 → loses 2 electrons → Mg2+
- Oxygen (O): Group 16 → gains 2 electrons → O2−
Check your prediction against the periodic table
Not sure if your answer is right? Compare your prediction to a periodic table with charges or a chart of charges on periodic table for confirmation. You’ll notice that aluminium’s +3, magnesium’s +2, and oxygen’s −2 charges are consistent with the most common ions listed in these tables [reference]. The same method helps you find the zinc ion charge (Zn2+) and many others.
Ready to test yourself? Try predicting the ionic charge for sodium, sulfur, or chlorine using the steps above. The more you practice, the more natural reading periodic table charges will become—and the easier it will be to write correct formulas for any ionic compound.
Next, we’ll explore why aluminium prefers to lose exactly three electrons—and what makes the +3 state so stable compared to other possibilities.
Why Aluminium Settles at +3
Successive Ionization Energies and the Al3+ Outcome
Sounds complex? Let’s break it down. When you look at the periodic table and wonder, “What is the charge of Al?” or “What charge does aluminum have?” the answer is almost always +3. But why? The secret lies in how aluminium atoms lose electrons and what makes that +3 state so stable compared to +1 or +2.
Imagine peeling layers off an onion. The first three electrons lost by aluminium are the outermost—its valence electrons. Removing these is relatively straightforward for a metal like aluminium, which sits in group 13. When these three electrons are gone, the atom reaches a stable, noble-gas-like core. That’s why the aluminum loss or gain of electrons is nearly always a loss of three.
Aluminium stops at +3 because the next electron would come from a much more tightly held inner shell.
Why Removing a Fourth Electron Is Unfavorable
Here’s the key: after aluminium loses its three valence electrons, the next available electron is buried deep in an inner shell, close to the nucleus and shielded from outside influences. Trying to remove a fourth electron would require breaking into this stable, tightly bound shell—a process that’s energetically very unfavorable. That’s why you never see a +4 aluminum ion in ordinary chemistry.
- First three electrons: Easily lost, emptying the 3s and 3p orbitals.
- Fourth electron: Would come from the 2p shell, which is much more stable and much harder to remove.
This is a classic example of the trend across the periodic table: metals lose their outer electrons until they reach a stable core, then stop. The ionization of aluminum fits this pattern perfectly [reference].
Metal Stability Through Electron Loss
So, does aluminum have a fixed charge? In practice, yes: the charge of an aluminum ion is almost always +3. While rare compounds exist where aluminum may appear as +1 or +2, these are exceptions and not the rule in real-world chemistry. That’s why, when you ask “what is the charge for aluminum in most compounds?” the answer is a reliable +3.
How many electrons does aluminum gain or lose? It loses three—never gains—because it’s a metal, and metals tend to shed electrons to reach a stable state. This is why the aluminum ionic charge is so predictable in everything from aluminum oxide (Al2O3) to aluminum chloride (AlCl3).
- +3 is the standard, stable charge for aluminum in ionic compounds.
- Loss of three electrons aligns with its metallic character and group 13 position.
- Al3+ is found in nearly all common aluminum salts and coordination complexes.
In summary, what is the charge of Al? It’s +3—because after those three electrons are gone, the atom is content, and chemistry “stops” there. This energetic logic is why aluminium ionic charge is so reliable, and why you’ll see the +3 ion everywhere in both nature and industry.
Next, you’ll see how this fixed charge translates into real-world formulas, and how to balance charges to write stable compounds with aluminium ions.
Charge Balancing to Write Aluminium Compounds
From Al3+ to Compound Formulas: Naming Ionic Compounds in Action
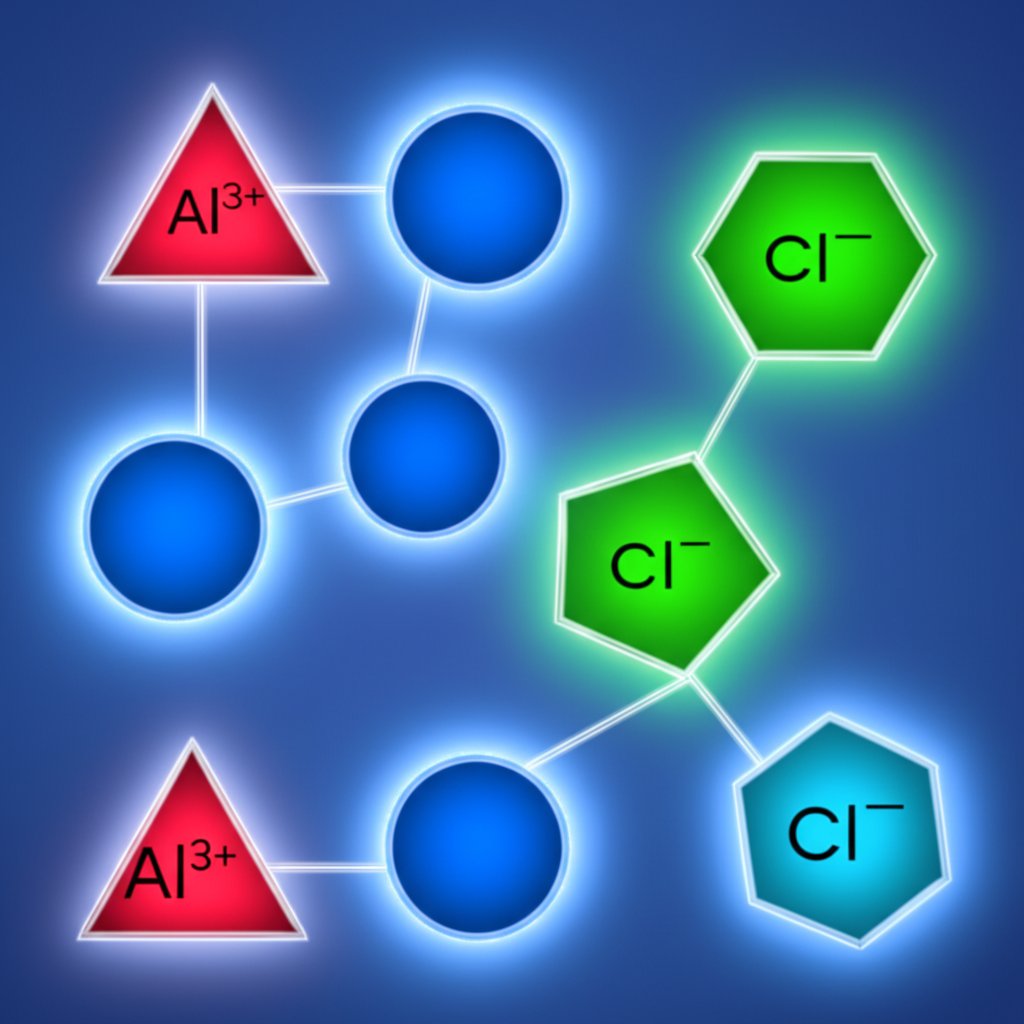
When you hear about the aluminium ionic charge, what does it mean for real chemical compounds? Let’s break it down with practical examples and a simple method for writing formulas that are always balanced and correct. Imagine you’re handed Al3+ ions and told to pair them with common anions—how do you know what the final formula should be? The answer is all about balancing ionic charges so the total positive equals total negative. Let’s see how it works, step by step.
Write the Half-Reaction for Aluminium
Start with the fundamental process: aluminium loses three electrons to form its ion.
Al → Al3+ + 3e−
This +3 charge is what you’ll use when pairing aluminium with other ions in naming ionic compounds. The key is to ensure that the sum of all charges in the compound equals zero—nature always prefers neutrality!
Balance Charges to Build Stable Salts
Let’s walk through four classic examples using aluminium’s +3 charge with several important anions. For each, we’ll see how to combine ions to reach a neutral formula, referencing the formulas for ionic compounds and standard classroom practice:
| Cation | Anion | Charges | Balanced Formula | Notes |
|---|---|---|---|---|
| Al3+ | O2− | +3, −2 | Al2O3 | 2 Al3+ (2 × +3 = +6), 3 O2− (3 × −2 = −6) |
| Al3+ | Cl− | +3, −1 | AlCl3 | 3 Cl− needed for charge balance |
| Al3+ | NO3− | +3, −1 | Al(NO3)3 | 3 nitrate ions (no3 ion charge is −1) for neutrality |
| Al3+ | SO42− | +3, −2 | Al2(SO4)3 | 2 Al3+ (+6), 3 sulfate ions (sulfate ion charge is −2, total −6) |
Let’s look at the logic behind these formulas:
- Al2O3: Two Al3+ ions (+6) and three O2− ions (−6) balance perfectly.
- AlCl3: Three chloride ions (chloride charge is −1) are needed to neutralize one Al3+.
- Al(NO3)3: Three nitrate ions (charge of nitrate is −1) balance one Al3+; parentheses show three whole nitrate groups.
- Al2(SO4)3: Two Al3+ (+6) and three sulfate ions (sulfate ion charge is −2, total −6) for neutrality.
Balancing Tips for Ionic Charges
- Always match the total positive charge to the total negative charge.
- Use the lowest whole-number ratio for each ion (reduce subscripts if possible).
- For polyatomic ions (like nitrate or sulfate), use parentheses if more than one is needed: Al(NO3)3, Al(OH)3.
- Check your work: sum of all ionic charges in the formula must be zero.
Want to try more? Practice with other polyatomic ions from standard tables—like pairing Al3+ with OH− (hydroxide charge is −1, giving Al(OH)3), or with PO43− (phosphate ion charge is −3, making AlPO4). For every case, the method stays the same: balance the ionic charges, then write the simplest formula.
Now that you’ve seen how to build and balance these formulas, you’re ready to distinguish between similar-sounding concepts like ionic charge, oxidation number, and formal charge. Let’s clear up these common mix-ups in the next section.
Avoiding Common Charge Concept Mix Ups
Ionic Charge vs. Oxidation Number vs. Formal Charge
When you’re learning about the aluminium ionic charge, it’s easy to get tripped up by similar terms—especially when textbooks and teachers throw around phrases like oxidation number and formal charge. Sounds complex? Let’s break down each concept in plain English and show you how to spot the difference, using aluminium as our guide.
| Concept | What it Measures | How It’s Assigned | Example with Al | When to Use |
|---|---|---|---|---|
| Ionic Charge | Actual net charge on an atom after gaining or losing electrons | Count electrons lost (positive) or gained (negative) compared to the neutral atom | Al3+ in AlCl3 has an ionic charge of +3 | When discussing ions in salts or solutions; key for writing formulas and balancing charges |
| Oxidation Number | Formal bookkeeping tool to track electron shifts in compounds | Assign all bonding electrons to the more electronegative atom; for simple ions, matches ionic charge | Al in AlCl3 has an oxidation number of +3 (same as ionic charge here) Al in Al2O3 is also +3 |
Used in redox reactions, naming, and electron accounting |
| Formal Charge | Hypothetical charge if bonding electrons are shared equally | Divide all bonds evenly, then compare to valence electrons in the free atom | Rarely applies to simple ionic compounds like AlCl3; more relevant in covalent molecules or polyatomic ions | Used when drawing Lewis structures to identify the most stable arrangement |
Simple Examples Using Aluminium
- In AlCl3: Aluminium’s ionic charge is +3, matching its oxidation number. Chloride ions each have a charge and oxidation number of -1.
- In Al2O3: Each aluminium atom has an ionic charge of +3 and an oxidation number of +3. Each oxygen is -2 for both.
- Formal charge: For these ionic compounds, formal charge isn’t usually discussed. It’s more relevant for covalent structures or polyatomic ions like sulfate or nitrate, where electron sharing is not as clear-cut.
When Each Concept Matters
Imagine you’re asked how to find a oxidation number for aluminium in a compound. For simple ions, the oxidation number and ionic charge are identical. But in covalent or complex ions, these numbers can diverge. Formal charge, meanwhile, is a tool chemists use when drawing Lewis structures to decide which structure is most likely, based on the idea of "equal sharing" of electrons.
Here’s how these ideas fit together when using a table of elements ionic charges or a periodic table with cations and anions:
- Ionic charge: Use for writing formulas, predicting compound ratios, and balancing reactions. Check the charges periodic table for quick reference.
- Oxidation number: Use for redox reactions, systematic naming, and understanding electron transfer.
- Formal charge: Use when comparing possible Lewis structures, especially for polyatomic ions and covalent molecules.
Common Pitfalls to Avoid
- Don’t confuse formal charge with the true ionic charge in ionic compounds—they may not match.
- Remember: oxidation number is a formalism, not a real charge, except for simple ions.
- Always check the sum of oxidation numbers in a compound: it must equal the overall charge of the molecule or ion (source).
Now that you can distinguish between these charge concepts, you’re ready to see how aluminium’s charge plays out in real-world applications and industrial materials. Next, let’s explore how Al3+ shows up in everything from water treatment to manufacturing, and why knowing these differences matters for chemistry in action.

Real World Uses of Aluminium Ionic Charge
From Ions to Materials: Where Al3+ Shows Up
When you understand the aluminium ionic charge, you begin to see its fingerprints everywhere—from the water you drink to the car you drive. But how does that +3 charge actually shape aluminum’s real-world behavior? Let’s break down the key ways this chemistry translates into everyday applications, and why the difference between alum vs aluminium matters in both science and industry.
- Shaoyi Metal Parts Supplier — Automotive aluminum extrusion parts: In manufacturing, the +3 ionic charge is foundational for aluminum’s corrosion resistance and suitability for anodizing. Shaoyi’s expertise leverages this principle to deliver high-performance, precisely engineered automotive parts, where controlled surface treatments and alloy selection depend on a deep understanding of Al3+ chemistry.
- Corrosion passivation and protective oxide: Have you ever wondered, "Does aluminum rust?" or "Can aluminum rust?" Unlike iron, aluminum doesn’t rust in the traditional sense. Instead, when exposed to air or water, it instantly forms a thin, stable layer of aluminum oxide (Al2O3) on its surface. This passivation layer is directly linked to the aluminum ion’s +3 charge—Al3+ binds strongly with oxygen, creating a barrier that protects the underlying metal from further corrosion. This is why aluminum structures last so long, even in harsh environments.
- Water treatment and flocculation: In municipal water plants, aluminum salts like aluminum sulfate are added to remove impurities. The Al3+ ions act as powerful coagulants, binding with suspended particles and causing them to settle out—making water clearer and safer to drink. You’ll often see the term "alum block" used for these coagulants. The distinction between alum vs aluminium is crucial here: "alum" refers to a specific class of aluminum-containing compounds, while "aluminium" is the pure metal or its simple ions [reference].
- Material selection and surface finishing: In industries ranging from aerospace to electronics, knowledge of aluminum ions informs choices about alloys, coatings, and treatments. For example, anodizing—an electrochemical process—thickens the natural oxide layer, boosting durability and appearance. This relies on the high reactivity and +3 charge of aluminum ions at the surface.
- Alumina density and advanced materials: The density and structure of alumina (Al2O3)—a ceramic made from aluminum ions—are critical in applications like cutting tools, catalysts, and even as a substrate for microelectronics. The +3 charge leads to tightly packed, stable ionic lattices, giving alumina its hardness and thermal stability.
Corrosion Resistance: Why Aluminum Passivates, Not Rusts
Imagine you’re comparing steel and aluminum outdoors. Steel forms flaky rust that eats away at the metal, but aluminum develops a tough, invisible oxide shield. This is because Al3+ ions at the surface grab oxygen atoms, locking them into a dense, protective layer. The result: aluminum’s corrosion resistance is one of its greatest assets, and why it’s so widely used in everything from beverage cans to skyscraper cladding.
Manufacturing Implications: From Extrusions to Everyday Objects
In manufacturing, understanding the aluminium ionic charge isn’t just academic—it shapes real decisions about materials and processes. For instance, automotive engineers rely on properties like alumina density and the behavior of aluminum ions to select alloys that balance strength, weight, and corrosion resistance. Surface treatments such as anodizing or painting are designed to enhance or modify the natural oxide layer, all thanks to the predictable chemistry of Al3+.
So next time you see an aluminum extrusion, a water treatment facility, or even a simple alum block, remember: the +3 charge of aluminum ions is at the heart of its performance. Whether you’re weighing alum vs aluminium for a specific application or choosing a supplier for precision parts, understanding this core chemical property will help you make smarter, more informed decisions.
Up next, you’ll get hands-on practice applying what you’ve learned—predicting charges and writing formulas for real-world compounds involving aluminum ions.
Hands on Practice with Aluminium Ions
Practice Set: Predict Charges and Formulas
When you’re learning about ionic charges, nothing beats hands-on practice. Below you’ll find a series of problems designed to reinforce what you’ve learned about the aluminium ionic charge and how to use it to build real-world chemical formulas. These problems will help you answer common questions like “what is the charge of an aluminum ion?” and “how do I write a balanced formula for an aluminum compound?”
-
State the ionic charge of aluminium.
What is the charge of aluminium when it forms an ion? -
Write the formula for Al3+ with Cl−.
Predict the correct formula for a compound between an aluminum ion and a chloride ion. -
Write the formula for Al3+ with NO3−.
Predict the formula for a compound formed by an aluminum ion and a nitrate ion. -
Write the formula for Al3+ with SO42−.
Predict the balanced formula for a compound containing an aluminum ion and a sulfate ion. -
Write the formula for Al3+ with O2−.
Predict the correct formula for a compound made from aluminum and oxide ions. -
Challenge: Balance overall charges in a reaction summary line.
Write a balanced summary for the reaction between aluminum ions and sulfate ions, showing how charges are balanced in the formula.
Total positive charge must equal total negative charge in the final formula.
Worked Solutions for Al3+ Pairings
-
State the ionic charge of aluminium.
The answer to “what is the charge of an aluminum ion” is +3. In chemical notation, this is written as Al3+. This means that when you predict the charge that an aluminum ion would have, you simply look for +3, just as you’d look for the charge of a potassium ion (K+) as +1. -
Write the formula for Al3+ with Cl−.
To balance the charges, you need three chloride ions (Cl−) for every aluminum ion (Al3+). The formula is AlCl3. This ensures the total charge is zero: (+3) + 3×(−1) = 0. -
Write the formula for Al3+ with NO3−.
Again, three nitrate ions (NO3−) are needed to balance one aluminum ion. The correct formula is Al(NO3)3. Parentheses are used because more than one polyatomic ion is present. -
Write the formula for Al3+ with SO42−.
Here, two aluminum ions (2 × +3 = +6) and three sulfate ions (3 × −2 = −6) are needed for a neutral compound. The balanced formula is Al2(SO4)3. -
Write the formula for Al3+ with O2−.
Two aluminum ions (2 × +3 = +6) and three oxide ions (3 × −2 = −6) give a neutral compound. The formula is Al2O3. This is the main component of alumina ceramics. -
Challenge: Balance overall charges in a reaction summary line.
Combine two Al3+ ions and three SO42− ions:- 2 × (+3) = +6 (from aluminum ions)
- 3 × (−2) = −6 (from sulfate ions)
- +6 + (−6) = 0 (neutral overall)
The balanced formula is Al2(SO4)3. This mirrors the balancing logic used for the charge of a potassium ion (K+) paired with a sulfate ion (K2SO4).
Try These Before Checking Answers
- What is the charge on an aluminum ion? (Al3+)
- What charge is aluminum in AlCl3? (+3)
- Predict the charge that an aluminum ion would have if it lost three electrons. (+3)
- How would you balance the formula for aluminum phosphate, knowing phosphate’s charge is −3? (AlPO4)
Mastering these ionic charges, from the charge of a potassium ion to the charge of an aluminum ion, will help you quickly predict and balance formulas for a wide range of compounds. If you’re ready for more, the next section will summarize the key takeaways and point you to reliable resources for deeper learning and practice.
Key Takeaways and Trusted Resources
Key Takeaways to Remember about Al3+
When you step back and look at the big picture, the chemistry of aluminium’s ionic charge is refreshingly predictable—and extremely useful. Here are the three core lessons to lock in:
- Aluminium typically forms Al3+ ions: The aluminium charge is almost always +3 in compounds, reflecting its position in group 13 of the periodic table and its tendency to lose three valence electrons.
- Ionic charges balance to create neutral formulas: Whether you’re building Al2O3, AlCl3, or Al(NO3)3, the total positive and negative charges always add up to zero. This fundamental principle is the backbone of writing and checking chemical formulas.
- The +3 state reflects both valence and energetic stability: Aluminium’s +3 ionic charge arises because removing a fourth electron would break into a stable inner shell, making +3 the favored—and most common—state in real-world chemistry.
Aluminium’s most common ionic charge is +3.
Resources to Go Deeper
Ready to reinforce your understanding or put your knowledge into practice? Here’s a curated list of resources to keep learning, from classroom basics to advanced manufacturing insights:
- Shaoyi Metal Parts Supplier — Automotive aluminum extrusion parts: Discover how the fundamental +3 aluminum charge underpins surface behavior, anodizing, and corrosion resistance in real-world automotive components. This is a practical bridge between chemical theory and manufacturing excellence, showing how knowledge of Al3+ translates into precise engineering and material selection.
- Consult a periodic table with charges: For instant reference, use a periodic table with ion charges to check the most common ionic states of any element. These tables are invaluable for students, teachers, and professionals who need to confirm the periodic table of charges at a glance. Resources like this ThoughtCo guide provide printable versions and helpful explanations.
- Review standard texts for oxidation number methods: For a deeper dive into the differences between ionic charge, oxidation number, and formal charge, classic chemistry textbooks and online modules are ideal for mastering these concepts in context.
From Classroom to Shop Floor: Why This Knowledge Matters
Imagine you’re moving from a chemistry class to a design meeting for a new automotive part. The ability to predict and balance the aluminium ionic charge isn’t just an academic skill—it’s a real advantage in materials selection, process engineering, and troubleshooting. Whether you’re reading a periodic table of elements with charges for a homework problem or consulting a periodic table with ion charges for a manufacturing project, these tools keep your decisions grounded in reliable science.
Keep these core ideas in mind, use trusted references, and you’ll find that the +3 aluminium charge is your key to understanding, predicting, and applying chemistry in both the lab and the real world.
Frequently Asked Questions About Aluminium Ionic Charge
1. What is the charge of an aluminum ion and why does it form this charge?
The charge of an aluminum ion is +3, written as Al3+. This occurs because aluminum, found in group 13 of the periodic table, loses its three valence electrons to achieve a stable electron configuration. This +3 charge is the most stable and common state for aluminum in compounds, making it highly predictable in chemical reactions and formula writing.
2. How can you predict the ionic charge of aluminium using the periodic table?
To predict aluminium's ionic charge, locate it in group 13 of the periodic table. Elements in this group typically lose their three outermost electrons, resulting in a +3 charge. This trend is consistent across main-group metals and helps you quickly deduce the most likely charge for aluminium and similar elements.
3. Why does aluminium not form +1 or +2 ions in common compounds?
Aluminium does not commonly form +1 or +2 ions because removing only one or two electrons does not achieve the stable, noble-gas-like electron configuration. After losing three electrons, the remaining electrons are much more tightly bound, making further loss energetically unfavorable. As a result, the +3 state dominates in both natural and industrial contexts.
4. How does aluminium's +3 charge affect its real-world uses, such as in manufacturing or corrosion resistance?
Aluminium's +3 charge enables it to form a stable oxide layer (alumina) on its surface, providing excellent corrosion resistance. This property is leveraged in industries like automotive manufacturing, where companies such as Shaoyi use aluminium's chemistry for advanced surface treatments like anodizing, resulting in durable, lightweight components ideal for critical vehicle systems.
5. What is the difference between ionic charge, oxidation number, and formal charge for aluminium?
Ionic charge refers to the actual net charge on an aluminium ion after it loses electrons (+3 for Al3+). The oxidation number is a bookkeeping tool that often matches the ionic charge in simple ions but can differ in complex compounds. Formal charge is mainly used in covalent Lewis structures and may not reflect the true charge found in ionic compounds. Understanding these differences is key for accurate chemical analysis.
 Small batches, high standards. Our rapid prototyping service makes validation faster and easier —
Small batches, high standards. Our rapid prototyping service makes validation faster and easier —