Dự Đoán Điện Tích Ion Của Nhôm Giống Chuyên Gia—Và Nhận Biết Các Ngoại Lệ Quan Trọng
Bắt Đầu Với Ý Nghĩa Của Điện Tích Ion Al
Điện tích ion Al nghĩa là gì trong từ ngữ đơn giản
Bạn đã từng tự hỏi tại sao nhôm trong các hợp chất hầu như luôn xuất hiện dưới dạng Al 3+? Khái niệm về nhôm rất đơn giản nhưng mạnh mẽ: nó cho bạn biết số electron mà một nguyên tử nhôm đã mất hoặc thu được để tạo thành ion ổn định. Đối với nhôm, điện tích phổ biến nhất – và đáng tin cậy nhất – là +3. Điều đó có nghĩa là mỗi ion nhôm đã mất đi ba electron, tạo thành một cation mang điện tích 3+. Đây là lý do tại sao, khi bạn thấy thuật ngữ điện tích nhôm hoặc điện tích của nhôm trong hóa học, nó hầu như luôn đề cập đến Al 3+.
Vị trí của Al trong bảng điện tích tuần hoàn và lý do tại sao nó quan trọng
Khi bạn nhìn vào một bảng tuần hoàn với điện tích ion , bạn sẽ nhận thấy rằng các nguyên tố trong cùng một nhóm thường tạo thành các ion có cùng điện tích. Nhôm nằm ở nhóm 13 (đôi khi được gọi là nhóm IIIA), ngay sau magiê và trước silic. Xu hướng là gì? Các kim loại nhóm chính có xu hướng mất electron để phù hợp với số electron của khí hiếm gần nhất. Đối với nhôm, điều này có nghĩa là mất đi ba electron—do đó có điện tích +3. Mô hình dựa trên nhóm này là cách rút gọn để dự đoán điện tích mà không cần phải ghi nhớ từng nguyên tố riêng lẻ. Ví dụ, kim loại nhóm 1 luôn tạo thành ion +1, kim loại nhóm 2 tạo thành ion +2, và nhóm 13—bao gồm nhôm—tạo thành ion +3. Đây là cơ sở cho nhiều điện tích bảng tuần hoàn theo nhóm biểu đồ tham khảo.
| Nhóm | Điện tích điển hình |
|---|---|
| 1 (Kim loại kiềm) | +1 |
| 2 (Kim loại kiềm thổ) | +2 |
| 13 (Nhóm của Nhôm) | +3 |
| 16 (Chalcogen) | −2 |
| 17 (Halogen) | −1 |
Kiểm tra nhanh để xác nhận Al 3+trong các hợp chất phổ biến
Hãy tưởng tượng bạn đang làm việc với Al 2O 3(aluminum oxide) hoặc AlCl 3(nhôm clorua). Làm thế nào bạn biết nhôm là +3? Đó là vấn đề cân bằng điện tích. Oxy thường có điện tích −2, và clorua có điện tích −1. Trong Al 2O 3, hai ion Al 3++3 (tổng cộng +6) cân bằng với ba ion O 2−−2 (tổng cộng −6). Trong AlCl 3, một Al 3+ion cân bằng ba Cl −ion (tổng −3). Những mô hình này giúp dễ dàng nhận biết và xác nhận điện tích của Al trong các hợp chất thực tế.
- AL 3+hình thành bằng cách mất đi ba electron, phù hợp với cấu hình khí hiếm gần nhất.
- Đây là ion ổn định duy nhất phổ biến của nhôm, khiến việc dự đoán trở nên đơn giản.
- Xu hướng nhóm trên bảng tuần hoàn giúp bạn nhanh chóng xác định Al 3+mà không cần học thuộc lòng.
Ý chính: Nhôm ưa trạng thái +3 vì trạng thái này mang lại cho nó cấu hình electron ổn định, giống khí hiếm – khiến Al 3+trở thành ion phổ biến trong hầu hết các hợp chất.
Bằng cách hiểu các xu hướng này và cách bảng tuần hoàn các điện tích công việc, bạn sẽ có thể dự đoán nhôm và các đối tác của nó trong các hợp chất một cách tự tin. Trong các phần tiếp theo, bạn sẽ thấy cách kiến thức này liên kết với hóa học trong dung dịch nước, quy tắc đặt tên, và thậm chí là hiệu suất vật liệu trong thế giới thực.
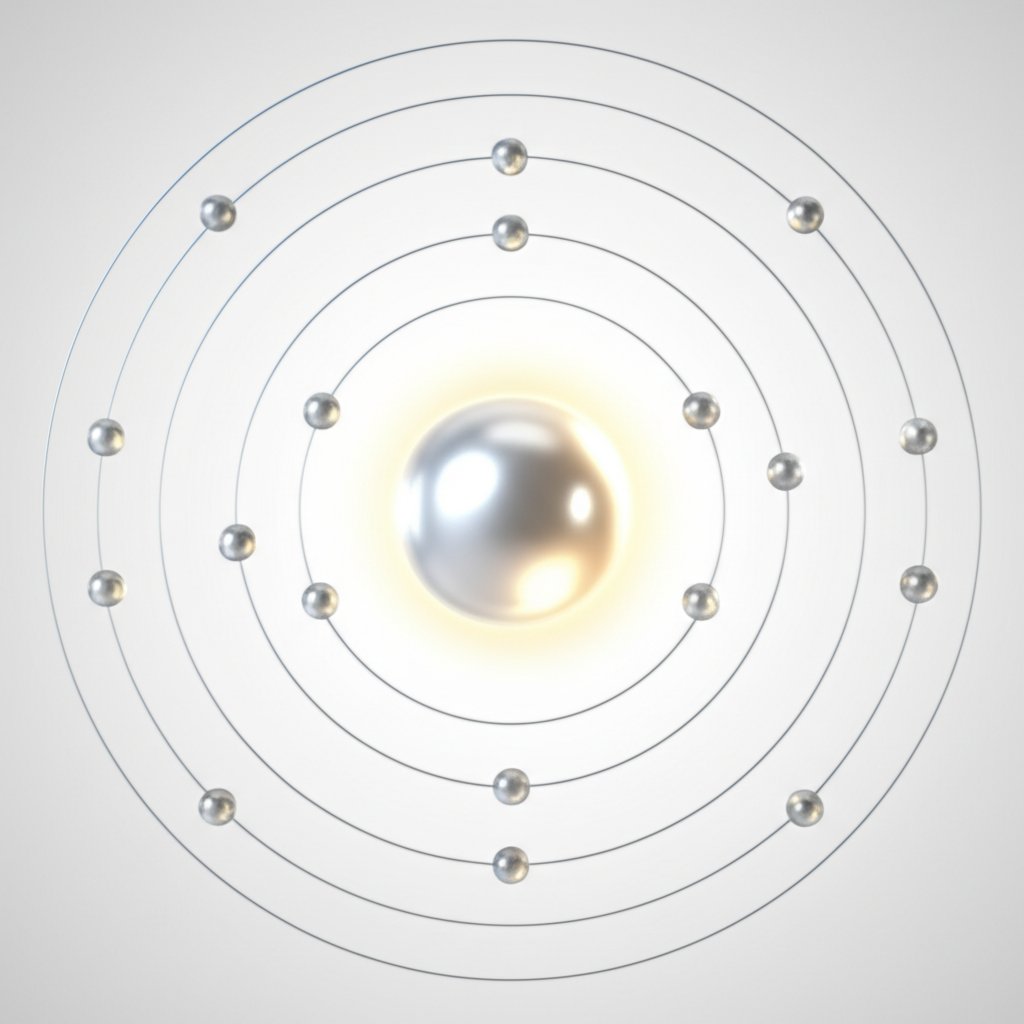
Cấu hình Electron Dẫn đến Al3 Plus
Electron hóa trị của Al và con đường tạo thành Al3+
Khi bạn lần đầu nhìn vào một nguyên tử nhôm, con đường dẫn đến điện tích +3 thông thường có vẻ bí ẩn. Nhưng nếu bạn phân tích theo cấu hình electron, logic này sẽ nhanh chóng trở nên rõ ràng. Nhôm có số nguyên tử là 13, nghĩa là nó chứa 13 electron khi ở trạng thái trung hòa. Cấu hình electron của nó được viết là 1s 22s 22P 63S 23P 1, hoặc viết gọn hơn, [Ne] 3s 23P 1. Ba electron trong các orbital 3s và 3p được xem là các electron hóa trị của nhôm – đây là những electron có khả năng cao bị mất trong các phản ứng hóa học.
Loại bỏ electron từng bước từ 3p rồi đến 3s
Nghe có vẻ phức tạp? Hãy tưởng tượng việc bóc từng lớp ra: các electron ở lớp ngoài cùng là những electron dễ bị loại bỏ nhất. Dưới đây là cách nguyên tử nhôm tạo thành một ion có điện tích +3:
- Loại bỏ electron 3p: Electron đơn lẻ trong orbital 3p bị mất trước tiên, để lại [Ne] 3s 2.
- Loại bỏ hai electron 3s: Tiếp theo, cả hai electron trong orbital 3s đều bị loại bỏ, kết quả là [Ne].
- Kết quả: Nguyên tử nhôm giờ đây đã mất tổng cộng ba electron, tạo thành một ion Al 3+có cấu hình electron giống với neon — một khí hiếm.
- Nhôm trung hòa: [Ne] 3s 23P 1
- Sau khi mất 1 electron: [Ne] 3s 2
- Sau khi mất thêm 2 electron: [Ne]
Quy trình từng bước này xuất phát từ mong muốn đạt được sự ổn định. số hóa trị của nhôm là 3, thể hiện ba electron mà nó có xu hướng mất đi để đạt được cấu hình khí hiếm. Khi nhôm tạo thành một ion với 10 electron, nó đã mất đi ba electron và trở thành Al 3+ (tham khảo) .
Tại sao nhôm là +3 mà không phải +1
Tại sao nhôm không dừng lại ở +1 hoặc +2? Câu trả lời nằm ở điện tích hạt nhân hiệu dụng và sự ổn định của lớp electron. Bằng cách mất đi cả ba electron hóa trị, điện tích ion của nhôm đạt được cấu hình lớp electron đã lấp đầy—giống với độ ổn định của neon. Nếu dừng lại ở +1 hoặc +2, các lớp electron sẽ vẫn còn trống một phần, kém ổn định hơn do sự phân bố electron không đều và hiệu ứng chắn yếu hơn. Đó là lý do tại sao điện tích ion của điện tích ion nhôm gần như luôn là +3 trong các hợp chất.
Khát khao đạt được cấu hình lớp electron đã lấp đầy, giống như khí hiếm khiến Al 3+trạng thái được ưu tiên áp đảo đối với các ion nhôm trong hóa học.
Hiểu những thay đổi electron này giúp bạn dự đoán và giải thích electron đối với nhôm trong các ngữ cảnh khác nhau. Tiếp theo, bạn sẽ thấy cách những mô hình này giúp bạn nhanh chóng dự đoán các điện tích đối với nhôm và các nguyên tố lân cận nó trên bảng tuần hoàn—và nhận biết các trường hợp ngoại lệ khi chúng xuất hiện.
Dự Đoán Điện Tích Ion Và Xử Lý Các Trường Hợp Ngoại Lệ
Dự đoán điện tích từ các mô hình tuần hoàn một cách nhanh chóng
Khi bạn nhìn vào bảng tuần hoàn với các điện tích , bạn sẽ nhận thấy một mô hình hữu ích: các nguyên tố trong cùng một nhóm (cột dọc) thường tạo thành các ion có cùng điện tích. Điều này làm cho bảng tuần hoàn ion một cách tắt mạnh mẽ để dự đoán điện tích ion có thể xảy ra của nhiều nguyên tố — đặc biệt là với các nguyên tố nhóm chính.
| Nhóm | Điện Tích Ion Thường Gặp |
|---|---|
| 1 (Kim loại kiềm) | +1 |
| 2 (Kim loại kiềm thổ) | +2 |
| 13 (Nhóm Bo, bao gồm Al) | +3 |
| 16 (Chalcogen) | −2 |
| 17 (Halogen) | −1 |
Ví dụ: điện tích nhóm 13 gần như luôn luôn là +3, do đó nhôm liên tục tạo thành Al 3+ion. Mô hình này được lặp lại trên toàn bảng tuần hoàn điện tích bảng tuần hoàn điện tích —Các nguyên tố nhóm 1 tạo thành +1, nhóm 2 tạo thành +2, và cứ tiếp tục như vậy. Khi bạn cần biết điện tích của Al là gì , bạn có thể nhanh chóng tham khảo vị trí nhóm của nó và tự tin dự đoán là +3 (tham khảo) .
Khi các ngoại lệ như Tl +ghi đè các quy tắc đơn giản
Nhưng còn các ngoại lệ thì sao? Mặc dù phần lớn các nguyên tố nhóm chính tuân theo các xu hướng này, có một vài điều bất ngờ — đặc biệt là khi bạn di chuyển xuống dưới một nhóm. Hãy lấy talium (Tl) trong Nhóm 13: mặc dù mức điện tích nhóm 13 thường là +3, talium thường tạo thành ion Tl +. Tại sao lại như vậy? Đó là do hiệu ứng cặp trơ (inert pair effect), trong đó các electron s ở mức năng lượng thấp hơn có xu hướng ít tham gia vào liên kết hơn khi nguyên tử trở nên nặng hơn. Kết quả là, talium có thể "giữ chặt" các electron s của nó, khiến trạng thái +1 ổn định hơn +3 trong nhiều hợp chất. Ngoại lệ này nhắc nhở chúng ta không nên quá phụ thuộc một cách mù quáng vào các xu hướng nhóm khi làm việc với các nguyên tố nặng hơn.
Cách xử lý các mức điện tích thay đổi của kim loại chuyển tiếp
Kim loại chuyển tiếp, được tìm thấy ở phần trung tâm của bảng tuần hoàn và mức điện tích (periodic table), nổi tiếng vì tính không thể đoán trước của chúng. Không giống như các kim loại nhóm chính, chúng có thể tạo thành các ion với nhiều mức điện tích khác nhau — ví dụ như Fe 2+và Fe 3+, hoặc Cu +và Cu 2+. Sự biến đổi này đồng nghĩa với việc bạn luôn nên kiểm tra một nguồn tham khảo hoặc bối cảnh của hợp chất khi làm việc với kim loại chuyển tiếp. Đừng giả định điện tích chỉ dựa vào vị trí nhóm.
- Xác định nhóm của nguyên tố: Sử dụng bảng tuần hoàn để tìm số nhóm.
- Áp dụng xu hướng nhóm: Dự đoán điện tích điển hình dựa trên nhóm (xem bảng phía trên).
- Kiểm tra các trường hợp ngoại lệ: Đối với các nguyên tố khối p nặng hơn (như Tl) hoặc kim loại chuyển tiếp, hãy tham khảo một nguồn đáng tin cậy.
Điện tích cố định +3 của nhôm đáng tin cậy hơn nhiều so với các điện tích thay đổi thấy ở kim loại chuyển tiếp - khiến nó trở thành một điểm tựa đáng tin cậy khi cân bằng các hợp chất ion.
Bằng cách nắm vững các mô hình này và nhận biết các ngoại lệ, bạn sẽ có thể sử dụng điện tích trên bảng tuần hoàn như một công cụ nhanh chóng và hiệu quả để xây dựng và kiểm tra công thức hóa học. Tiếp theo, bạn sẽ thấy cách những dự đoán này liên kết với hành vi trong thực tế của các ion nhôm trong nước và hơn thế nữa.
Hóa học trong dung dịch của Al3 +Và Thủy phân
Hexaaqua Al 3+và Trình tự thủy phân
Khi bạn hòa tan một muối nhôm như Al(NO 3)3trong nước, bạn không chỉ đơn thuần giải phóng các ion Al 3+thay vào đó, các cation nhôm ngay lập tức thu hút và liên kết với sáu phân tử nước, tạo thành phức chất ổn định phức hexaaqua [Al(H 2O) 6]3+. Ion này là bát diện, với số phối trí là 6—một đặc điểm chung của nhôm trong môi trường nước (tham khảo) .
Nhưng câu chuyện không dừng lại ở đó. Điện tích dương cao của Al 3+trạng thái điện tích này là yếu tố chính mang lại khả năng chống ăn mòn và chất lượng bề mặt hoàn thiện cho nhôm. Việc hợp tác với một nhà cung cấp kiểm soát được hóa học này ở mọi giai đoạn sẽ giúp các bộ phận của bạn có độ bền cao hơn và hoạt động tốt hơn. hydrolyse — tạo ra một chuỗi các ion mới như được minh họa dưới đây:
- Ở pH thấp: [Al(H 2O) 6]3+chiếm ưu thế.
- Khi pH tăng lên: Một ligand nước mất đi một proton, tạo thành [Al(H 2O) 5(OH)] 2+.
- Sự khử proton tiếp theo tạo ra [Al(H 2O) 4(OH) 2]+.
- Cuối cùng, Al(OH) 3(nhôm hydroxide) kết tủa ra ngoài.
- Ở pH cao: Al(OH) 4−(ion aluminat) được hình thành và hòa tan trở lại.
Chuỗi phản ứng này là một ví dụ điển hình về cách cation và anion tương tác với nhau trong nước, và lý do tại sao điện tích hydroxide rất quan trọng trong việc xác định các loài hiện diện ở mức pH cụ thể (nguồn) .
Tính lưỡng tính và Con đường dẫn đến Aluminat
Đây là lúc mọi thứ trở nên thú vị: Al(OH) 3đã lưỡng tính . Điều đó có nghĩa là nó có thể phản ứng với cả axit và bazơ. Trong dung dịch axit, nó hòa tan lại để tạo thành Al 3+(hoặc các dạng ngậm nước của nó). Trong dung dịch bazơ, nó tiếp tục phản ứng để tạo thành ion aluminat hòa tan, Al(OH) 4−. Hành vi kép này là đặc trưng của nhiều nhôm và rất quan trọng để hiểu tính hòa tan và kết tủa của chúng trong các môi trường khác nhau.
-
Các ligand phổ biến của Al 3+:
- Nước (H 2O)
- Hydroxide (OH −)
- Fluoride (F −)
- Sunfat (SO 42−)
- Axit hữu cơ (như citrat hoặc oxalat)
Hành vi này là lý do tại sao nhôm lại linh hoạt đến vậy trong xử lý nước, nhuộm màu, và thậm chí như một chất keo tụ — khả năng chuyển đổi giữa các dạng khác nhau tùy theo pH là chìa khóa cho hóa học của nó.
Al (Nhôm) 3+Điện Tích Ảnh Hưởng Thế Nào Đến Độ Hòa Tan
Vậy thì tất cả những điều này có ý nghĩa gì đối với độ hòa tan của ion nhôm các hợp chất? Trong điều kiện trung tính đến hơi kiềm, Al(OH) 3có độ hòa tan cực thấp và kết tủa ra — đây là cơ sở để loại bỏ nhôm khỏi nước. Nhưng trong điều kiện axit mạnh hoặc kiềm mạnh, nhôm vẫn ở dạng hòa tan như là [Al(H 2O) 6]3+hoặc Al(OH) 4−. Hành vi lưỡng tính này là lý do tại sao cation nhôm hóa học rất quan trọng trong các quy trình môi trường và công nghiệp.
Mật độ điện tích cao của Al 3+khiến nó trở thành một axit Lewis mạnh, thúc đẩy phản ứng thủy phân từng bước và sự hình thành nhiều dạng ion nhôm khác nhau trong dung dịch.
Hiểu được các biến đổi này sẽ giúp bạn dự đoán không chỉ những ion nào nhôm hiện diện ở các mức pH khác nhau, mà còn cách kiểm soát sự kết tủa, độ tan và phản ứng hóa học của chúng. Trong phần tiếp theo, bạn sẽ thấy những hành vi trong dung dịch này liên quan trực tiếp như thế nào đến các quy tắc đặt tên và mô hình công thức hóa học của các hợp chất nhôm trong các tình huống thực tế.
Quy Tắc Đặt Tên Và Mô Hình Công Thức Hóa Học Của Nhôm
Đặt tên đúng cho các hợp chất nhôm
Khi bạn nhìn thấy Al 3+trong một hợp chất, việc đặt tên cho nó khá đơn giản và dễ nhớ. Tên của ion nhôm trong hợp chất chỉ đơn giản là “ion nhôm”, vì nó chỉ tạo ra một loại điện tích phổ biến trong các hợp chất ion. Không cần phải gây nhầm lẫn hay thêm ký hiệu phụ — trừ khi bạn đang tuân theo một phong cách yêu cầu dùng chữ số La Mã để làm rõ nghĩa. Ví dụ, cả “aluminum chloride” và “aluminum(III) chloride” đều được chấp nhận, nhưng việc dùng chữ số La Mã là tùy chọn vì điện tích của nhôm luôn luôn là +3 trong các trường hợp này.
Cân bằng Al 3+với các anion phổ biến
Viết công thức hóa học cho các hợp chất chứa Al 3+tuân theo một tập hợp quy tắc rõ ràng: tổng điện tích dương phải cân bằng với tổng điện tích âm. Đây chính là cốt lõi của việc cân bằng điện tích hợp chất ion cân bằng điện tích hợp chất ion. Hãy xem cách kết hợp ion mang điện tích của nhôm với một số anion thường gặp nhất, bao gồm cả các anion đa nguyên tử như điện tích ion phosphate , điện tích ion acetate , và nồng độ nitrat :
| Công thức | Các ion thành phần | Tên | Ghi chú cân bằng điện tích |
|---|---|---|---|
| AL 2O 3 | 2 Al 3+, 3 O 2− | Oxit nhôm | 2×(+3) + 3×(−2) = 0 |
| AlCl 3 | 1 Al 3+, 3 Cl − | Nhôm clorua | 1×(+3) + 3×(−1) = 0 |
| AL 2(SO 4)3 | 2 Al 3+, 3 SO 42− | Axit sunfat nhôm | 2×(+3) + 3×(−2) = 0 |
| Al(NO 3)3 | 1 Al 3+, 3 NO 3− | Nhôm nitrat | 1×(+3) + 3×(−1) = 0 |
| Al(C 2H 3O 2)3 | 1 Al 3+, 3 C 2H 3O 2− | Nhôm acetate | 1×(+3) + 3×(−1) = 0 |
| AlPO 4 | 1 Al 3+, 1 PO 43− | Nhôm phosphat | 1×(+3) + 1×(−3) = 0 |
Lưu ý cách chọn chỉ số dưới để đảm bảo tổng điện tích dương và điện tích âm bằng không. Đối với các ion đa nguyên tử, nếu bạn cần nhiều hơn một, hãy luôn đặt ion trong dấu ngoặc trước khi thêm chỉ số dưới (ví dụ: Al(NO 3)3).
Khi nào cần bao gồm chữ số La Mã
Vì tên ion của nhôm là rõ ràng, bạn sẽ thường thấy từ “ion nhôm” mà không có chữ số La Mã. Tuy nhiên, một số sách giáo khoa hoặc tài liệu tham khảo vẫn có thể sử dụng “nhôm(III)” để nhấn mạnh điện tích +3, đặc biệt là trong bối cảnh mà các nguyên tố khác có thể có nhiều trạng thái oxy hóa. Với nhôm, điều này chủ yếu là một lựa chọn phong cách – chứ không phải là bắt buộc (xem nguồn) .
- Quên sử dụng dấu ngoặc đơn quanh các ion đa nguyên tử khi có nhiều hơn một ion như vậy, ví dụ, viết AlNO 33thay vì Al(NO 3)3
- Tính sai tổng điện tích và kết thúc bằng một công thức không cân bằng
- Nhầm lẫn điện tích của các ion đa nguyên tử phổ biến, ví dụ như điện tích ion phosphate (−3), điện tích ion acetate (−1), hoặc nồng độ nitrat (−1)
Quy tắc cơ bản: Luôn cân bằng tổng điện tích dương và điện tích âm – sử dụng tỷ lệ số nguyên nhỏ nhất cho công thức, và kiểm tra lại điện tích của ion đa nguyên tử cũng như dấu ngoặc đơn.
Với những quy ước và ví dụ này, bạn sẽ có thể tự tin viết và đặt tên cho bất kỳ hợp chất ion chứa nhôm nào một cách nhanh chóng. Tiếp theo, hãy xem các quy tắc đặt tên này liên quan như thế nào đến tác động thực tế của các ion nhôm trong vật liệu và quá trình hoàn thiện.
Tác Động Thực Tế Của Nhôm 3+Trong Vật Liệu Và Quá Trình Hoàn Thiện
Từ Al 3+đến Lớp Oxit và Quy Trình Anot Hóa
Khi bạn nghĩ về độ bền và hiệu suất của các bộ phận bằng nhôm, điện tích ion nhôm không chỉ đơn thuần là một khái niệm trong sách giáo khoa – đó chính là nền tảng cho cách mà nhôm hoạt động trong môi trường thực tế. Bạn đã từng để ý rằng bề mặt nhôm phát triển một lớp mỏng bảo vệ gần như ngay lập tức chưa? Đó chính là kết quả của Al 3+ion phản ứng với oxy để tạo thành một lớp oxit ổn định. Lớp thụ động hóa tự nhiên này bảo vệ kim loại bên dưới khỏi sự ăn mòn tiếp tục và là yếu tố chính giúp nhôm được sử dụng rộng rãi trong kỹ thuật và sản xuất.
Nhưng điều gì xảy ra khi bạn cần lớp bảo vệ tốt hơn hoặc một lớp hoàn thiện bề mặt cụ thể? Đó là lúc anodizing phương pháp anod hóa (anodizing) được áp dụng. Anod hóa là một quá trình điện hóa có kiểm soát, làm dày thêm lớp oxit một cách chủ ý bằng cách thúc đẩy sự hình thành oxit nhôm ngậm nước bằng dòng điện bên ngoài. Quá trình này dựa vào sự di chuyển và biến đổi của nhôm ion trên bề mặt—khả năng tồn tại dưới dạng Al 3+của nhôm càng mạnh thì lớp oxit thu được càng bền (tham khảo) .
- AL 3+các ion di chuyển đến bề mặt dưới tác dụng của điện áp
- Chúng phản ứng với nước và oxy để tạo thành một lớp oxit dày đặc, có tính bảo vệ
- Lớp oxit được thiết kế này có khả năng chống lại sự ăn mòn, mài mòn và hư hại do tác động môi trường
Hãy tưởng tượng bạn đang thiết kế một bộ phận ô tô tiếp xúc với muối đường, độ ẩm hoặc nhiệt độ cao — nếu không có lớp oxit được tạo ra bởi ion này, bộ phận đó sẽ nhanh chóng bị hư hỏng. Đó là lý do tại sao việc hiểu rõ nhôm mang điện tích gì không chỉ đơn thuần là một thông tin hóa học thú vị, mà còn là một mối quan tâm thiết kế thực tế.
Các tác động thiết kế đối với bộ phận nhôm ép đùn
Bây giờ, hãy kết nối các điểm này với quá trình ép đùn và hoàn thiện. Khi bạn chỉ định một hợp kim nhôm hoặc dạng profile cho một ứng dụng quan trọng, bạn không chỉ đang xem xét hình dạng hay độ bền — bạn còn đang suy nghĩ về cách bề mặt sẽ hoạt động dưới tác động của các lực căng trong thực tế. Xu hướng của Al 3+tạo thành oxit ổn định đồng nghĩa với việc các bộ phận ép đùn có thể được thiết kế với các loại lớp anod hóa khác nhau, mỗi loại mang lại hiệu suất riêng biệt:
- Cấp vật liệu: Thành phần hợp kim ảnh hưởng đến sự hình thành oxit và khả năng chống ăn mòn
- Xử lý bề mặt: Loại I (axit cromic), Loại II (lớp phủ trong suốt), và Loại III (anod cứng) mang lại độ bền và vẻ ngoài khác nhau
- Kiểm soát dung sai: Quy trình anodizing có thể được thiết kế để duy trì chính xác các kích thước cho các bộ phận hiệu suất cao
- Nhôm có thể phân cực: Khả năng kiểm soát điện tích bề mặt và độ dày của lớp oxit là yếu tố quan trọng đối với các ứng dụng yêu cầu cách điện hoặc dẫn điện
Đối với các ứng dụng ô tô, hàng không hoặc kiến trúc, sự kết hợp đúng đắn giữa hợp kim và lớp hoàn thiện bề mặt—có nền tảng từ điện tích ion nhôm —sẽ đảm bảo bộ phận tồn tại lâu dài, trông đẹp mắt và hoạt động đúng như mong đợi. Vẫn còn thắc mắc liệu "nhôm nhận hay mất electron"? Trong tất cả các quá trình này, nhôm mất electron để tạo thành cation, thúc đẩy toàn bộ chu trình oxy hóa và bảo vệ.
Các Đối Tác Cung Ứng Hiểu Rõ Hành Vi Ion Trong Quy Trình Hoàn Thiện
Việc lựa chọn một nhà cung cấp thực sự hiểu rõ về hóa học phía sau cation hoặc anion nhôm sự chuyển đổi có thể quyết định sự thành công hay thất bại của dự án bạn. Dưới đây là bảng so sánh các nhà cung cấp giải pháp cho các bộ phận nhôm đùn ép, tập trung vào chuyên môn của họ trong hoàn thiện bề mặt và kiểm soát chất lượng:
| Nhà cung cấp | Chuyên môn Hoàn Thiện Bề Mặt | Thực hành chất lượng | Phạm Vi Dịch Vụ |
|---|---|---|---|
| Shaoyi (linh kiện đùn ép nhôm) | Anode hóa tiên tiến, kiểm soát chính xác lớp oxit, kỹ thuật bề mặt đạt tiêu chuẩn ô tô | Chứng nhận IATF 16949, truy xuất nguồn gốc toàn bộ quy trình, DFM/SPC/CPK cho các kích thước quan trọng | Giải pháp trọn gói: thiết kế, chế tạo mẫu, sản xuất hàng loạt, giao hàng toàn cầu |
| Fonnov Aluminium | Xử lý anode tùy chỉnh, sơn phủ bột, hoàn thiện kiến trúc và kỹ thuật | Tuân thủ tiêu chuẩn quốc gia và quốc tế, phương châm chất lượng là hàng đầu | Thiết kế, đùn ép, gia công, hoàn thiện phục vụ đa ngành công nghiệp |
Khi đánh giá đối tác, hãy cân nhắc:
- Các cấp độ vật liệu và lựa chọn hợp kim cho ứng dụng của bạn
- Chuyên môn trong các phương pháp xử lý bề mặt (anodizing, sơn tĩnh điện, v.v.)
- Khả năng đáp ứng các dung sai chặt chẽ và yêu cầu bề mặt quan trọng
- Chứng nhận chất lượng và tính minh bạch trong quy trình
- Kinh nghiệm trong việc giảm thiểu ăn mòn và kỹ thuật lớp oxit
Nhận xét quan trọng: The Al 3+trong kỹ thuật bề mặt, bạn sẽ được trang bị tốt hơn để xác định thông số kỹ thuật, tìm nguồn cung ứng và bảo trì các bộ phận nhôm hiệu suất cao. Phần tiếp theo sẽ giới thiệu các công cụ và quy trình thực tế để dự đoán và áp dụng các khái niệm điện tích này trong các dự án của bạn.
Bằng cách hiểu rõ vai trò của điện tích ion nhôm trong lĩnh vực kỹ thuật bề mặt, bạn sẽ được trang bị tốt hơn để xác định, lựa chọn và bảo trì các bộ phận nhôm hiệu suất cao. Tiếp theo, hãy khám phá các công cụ và quy trình thực tế để dự đoán và áp dụng các khái niệm điện tích này vào các dự án của bạn.
Công Cụ Và Quy Trình Làm Việc Để Dự Đoán Điện Tích Chính Xác
Xây dựng Quy trình Dự đoán Điện tích Tín cậy
Đã bao giờ bạn nhìn vào một công thức hóa học và tự hỏi, "Làm thế nào để biết điện tích của mỗi nguyên tố—đặc biệt là nhôm?" Bạn không đơn độc đâu. Việc dự đoán điện tích ion đúng thật sự có thể khiến người ta choáng ngợp, nhưng với một bảng tuần hoàn các nguyên tố có ghi điện tích được trình bày rõ ràng và một vài thói quen thông minh, bạn sẽ nhanh chóng làm chủ kiến thức này. Bí quyết là hãy dùng bảng tuần hoàn như điểm tham chiếu đầu tiên, sau đó xác nhận chi tiết đối với các ion đa nguyên tử và các trường hợp đặc biệt khi bạn gặp phải.
| Nhóm | Điện tích phổ biến |
|---|---|
| 1 (Kim loại kiềm) | +1 |
| 2 (Kim loại kiềm thổ) | +2 |
| 13 (nhóm của Nhôm) | +3 |
| 16 (Chalcogen) | −2 |
| 17 (Halogen) | −1 |
Bảng đơn giản này phản ánh bố cục bạn thường thấy trên hầu hết các bảng tuần hoàn có ghi điện tích . Đối với nhôm, hãy luôn dự đoán là +3—điều này khiến nó trở thành một trong những cation dễ nhớ nhất trên bảng tuần hoàn.
Sử dụng Xu hướng Nhóm và Xác nhận các Ion Đa nguyên tử
Khi bạn đã sẵn sàng giải quyết các công thức phức tạp hơn, đừng chỉ dựa vào trí nhớ. Bảng bảng tuần hoàn với cation và anion sẽ giúp bạn với các nguyên tố nhóm chính, nhưng với ion đa nguyên tử, bạn cần một danh sách đã được xác minh. Dưới đây là một số ion thông dụng nhất mà bạn sẽ gặp, cùng điện tích của chúng:
| Tên | Công thức | Sạc |
|---|---|---|
| Nitrat | Không 3− | −1 |
| Sulfat | Vậy 42− | −2 |
| Photphat | Th 43− | −3 |
| Acetate (Axetat) | C 2H 3O 2− | −1 |
| Hydroxide | OH − | −1 |
| Cacbonat | C 32− | −2 |
| Amoni | Nh 4+ | +1 |
Hãy luôn giữ sẵn một bản in danh sách các ion này bên cạnh khi bạn đang giải bài tập hoặc viết báo cáo thí nghiệm. Để xem danh sách đầy đủ, hãy tham khảo tài liệu tham khảo về ion đa nguyên tử .
Viết Công Thức Cân Bằng Nhanh Chóng và Chính Xác
Khi bạn đã biết các điện tích, việc viết công thức đúng sẽ trở thành việc cân bằng tổng điện tích dương và âm sao cho tổng bằng không. Dưới đây là quy trình nhanh để luôn thực hiện chính xác:
- Tìm mỗi nguyên tố hoặc ion trên bảng tuần hoàn các nguyên tố và điện tích hoặc danh sách ion đa nguyên tử của bạn.
- Viết các ký hiệu ion cùng với điện tích của chúng (ví dụ: Al 3+, nên 42−).
- Xác định tỷ lệ ion thấp nhất mà cân bằng được điện tích về không.
- Viết công thức thực nghiệm, sử dụng dấu ngoặc đơn cho các ion đa nguyên tử nếu cần nhiều hơn một (ví dụ: Al 2(SO 4)3).
- Kiểm tra lại công việc của bạn: tổng các điện tích có bằng không không?
Ghi nhớ: "Al luôn hướng đến +3—hãy dùng bảng tuần hoàn, cân bằng điện tích, và bạn sẽ không bao giờ sai."
Bằng cách tuân theo quy trình này và sử dụng một bảng tuần hoàn có ghi điện tích làm mốc, bạn sẽ đơn giản hóa việc làm bài tập về nhà, chuẩn bị phòng thí nghiệm và cả việc giải quyết các bài toán kiểm tra. Hãy nhớ rằng, với điện tích của nhôm là bao nhiêu , câu trả lời luôn là +3—mọi lúc, trừ khi một ngoại lệ hiếm gặp được chỉ rõ.
Với những công cụ và quy trình thực tế này, bạn sẽ tiến dần từ việc ghi nhớ sang việc thực sự hiểu rõ các điện tích trong bảng tuần hoàn—và bạn sẽ sẵn sàng đối mặt với mọi thách thức liên quan đến việc đặt tên hoặc lập công thức hóa học tiếp theo.
Tổng hợp và các bước tiếp theo để sử dụng Al một cách tự tin 3+
Những điểm chính về Al 3+bạn Có Thể Tin Tưởng
Khi bạn lùi lại một bước và nhìn vào toàn cảnh, việc dự đoán nhôm trở thành một quy trình đơn giản và đáng tin cậy. Đây là lý do:
- Lập luận dựa trên bảng tuần hoàn: Vị trí của nhôm trong nhóm 13 cho thấy nó hầu như luôn tạo thành ion +3. Nếu bạn từng cảm thấy không chắc chắn về điện tích của nhôm là gì , hãy nhớ rằng xu hướng nhóm này chính là cách nhanh nhất dẫn đến câu trả lời đúng.
- Cấu hình electron: Bằng cách mất đi ba electron hóa trị, nhôm đạt được cấu trúc lõi khí hiếm—khiến Al 3+trở thành trạng thái ổn định và phổ biến nhất. Đây chính là câu trả lời cho câu hỏi “ nhôm tạo thành ion nào ?”
- Hóa học dự đoán được: Dù bạn đang cân bằng công thức, đặt tên hợp chất hay xem xét hiện tượng ăn mòn, bạn luôn có thể xác định Al 3+là dạng mặc định điện tích ion của nhôm .
- Nhôm hầu như luôn tạo thành cation +3—dễ dự đoán, ổn định và dễ nhận biết.
- AL 3+thúc đẩy hóa học nước, hình thành hợp chất, và chống ăn mòn.
- Kiến thức về điện tích này giúp bạn giải quyết các thách thức thiết kế, nguồn cung và giải quyết vấn đề trong thế giới thực.
Làm thế nào để áp dụng kiến thức này tiếp theo
Vậy làm sao biết được phí cho Al giúp bạn vượt ra ngoài lớp học? Hãy tưởng tượng bạn là:
- Thiết kế quy trình xử lý nước 3+thủy phân cho phép bạn kiểm soát lượng mưa và độ hòa tan.
- Viết công thức hóa học 3+là neo của bạn để cân bằng điện tích với anion thông thường.
- Xác định hoặc tìm nguồn các bộ phận bằng nhôm ép đùn - hiểu rõ điện tích của ion được hình thành từ nhôm giúp bạn hiểu tại sao màng oxit hình thành và cách xử lý anod hóa bảo vệ các bộ phận của bạn.
Nếu bạn từng không chắc chắn, hãy tự hỏi mình: Nhôm là cation hay anion trong ngữ cảnh này? Câu trả lời hầu như luôn là cation (Al 3+), và sự rõ ràng này sẽ giúp công việc của bạn nhanh chóng hơn - cho dù bạn đang chuẩn bị cho một bài kiểm tra hay thiết kế một sản phẩm mới.
| Khái niệm | Ví dụ | Ứng dụng |
|---|---|---|
| Vị trí nhóm 13 | Al tạo thành Al 3+ | Dự đoán tốc độ sạc |
| Mất electron đến [Ne] | Al: [Ne]3s 23P 1→ Al 3+: [Ne] | Giải thích độ ổn định |
| AL 3+trong nước | [Al(H 2O) 6]3+phức tạp | Hóa học trong dung dịch nước, phản ứng thủy phân |
| Hình thành lớp màng oxit | AL 3++ O 2−→ Al 2O 3 | Khả năng chống ăn mòn, anodizing |
Các nguồn tài liệu đề xuất để luyện tập và tìm hiểu
Đã sẵn sàng áp dụng kiến thức của bạn vào thực tế chưa? Đây là những bước tiếp theo bạn nên thực hiện:
- Shaoyi (linh kiện đùn ép nhôm) – Đối với các kỹ sư và nhà thiết kế đang tìm kiếm các bộ phận nhôm định hình hiệu suất cao, chống ăn mòn, Shaoyi nổi bật nhờ chuyên môn trong lĩnh vực anodizing, kỹ thuật màng oxit và hoàn thiện đạt tiêu chuẩn ô tô. Hiểu biết về hành vi ion của nhôm giúp họ tạo ra các bộ phận tốt hơn và bền lâu hơn.
- Hướng dẫn Hóa học Nhóm 13 – Củng cố kiến thức về xu hướng tuần hoàn, các ngoại lệ trong nhóm và logic điện tích trong bối cảnh cụ thể.
- Bảng tuần hoàn có điện tích – Tài liệu in có thể dùng để dự đoán điện tích và viết công thức nhanh chóng.
Dù bạn đang ôn thi hóa học hay lựa chọn vật liệu cho một sản phẩm mới, việc hiểu rõ nhôm có điện tích như thế nào sẽ là một kỹ năng bạn sẽ sử dụng nhiều lần. Và khi bạn cần các bộ phận được thiết kế để đạt độ bền tối đa, hãy tham khảo một nhà cung cấp như Shaoyi, người hiểu rõ khoa học đằng sau mọi bề mặt.
Điện tích ion của Al: Những câu hỏi thường gặp
1. Điện tích ion của nhôm là bao nhiêu và tại sao nó tạo thành Al3+?
Nhôm hầu như luôn tạo thành điện tích ion +3 vì nó mất đi ba electron hóa trị để đạt được cấu hình bền của khí hiếm. Điều này khiến Al3+ trở thành ion phổ biến nhất và ổn định nhất trong các hợp chất, làm cho việc dự đoán điện tích và viết công thức trở nên đơn giản hơn.
2. Làm thế nào tôi có thể nhanh chóng dự đoán điện tích của nhôm bằng bảng tuần hoàn?
Để dự đoán điện tích của nhôm, hãy xác định vị trí của nó trong Nhóm 13 của bảng tuần hoàn. Các nguyên tố nhóm chính trong nhóm này thường tạo thành cation +3, do đó điện tích của nhôm đáng tin cậy là +3. Xu hướng dựa trên nhóm này giúp bạn dự đoán điện tích mà không cần phải ghi nhớ từng nguyên tố riêng biệt.
3. Tại sao điện tích +3 của nhôm lại quan trọng trong các ứng dụng thực tế như anodizing?
Điện tích +3 của nhôm cho phép tạo thành một lớp oxit ổn định trên bề mặt, điều này rất quan trọng để chống ăn mòn và tăng độ bền. Tính chất này đóng vai trò then chốt trong các quá trình như anodizing, nơi lớp oxit được làm dày một cách có chủ đích để bảo vệ và cải thiện các bộ phận nhôm được sử dụng trong các ngành công nghiệp như sản xuất ô tô.
4. Điện tích ion của nhôm ảnh hưởng như thế nào đến hành vi của nó trong nước và các hợp chất?
Trong nước, Al3+ tạo thành các phức chất với các phân tử nước và trải qua phản ứng thủy phân, dẫn đến sự hình thành của nhiều loại ion nhôm khác nhau phụ thuộc vào pH. Điện tích mạnh của nó cũng thúc đẩy sự hình thành các hợp chất ion ổn định, với các công thức có thể dự đoán được dựa trên sự cân bằng điện tích với các anion thông thường.
5. Tôi nên lưu ý điều gì khi tìm nguồn cung cấp các bộ phận bằng nhôm cho các dự án liên quan đến hóa học ion?
Chọn các nhà cung cấp có chuyên môn về tính chất ion của nhôm và các công nghệ xử lý bề mặt tiên tiến. Ví dụ, Shaoyi cung cấp giải pháp ép đùn nhôm tích hợp, đảm bảo các thành phần có hóa tính bề mặt và độ bền tối ưu nhờ kiểm soát chính xác quá trình anodizing và hình thành lớp oxit.
 Sản xuất với số lượng nhỏ, tiêu chuẩn cao. Dịch vụ tạo nguyên mẫu nhanh của chúng tôi giúp việc kiểm chứng trở nên nhanh chóng và dễ dàng hơn —
Sản xuất với số lượng nhỏ, tiêu chuẩn cao. Dịch vụ tạo nguyên mẫu nhanh của chúng tôi giúp việc kiểm chứng trở nên nhanh chóng và dễ dàng hơn —
